A hierarchical approach to combine histological grade and immunohistochemical factors to identify high-risk luminal breast cancers
Felipe Andrés Cordero da Luz1,2a, Eduarda da Costa Marinho1b, Camila Piqui Nascimento1c, Lara de Andrade Marques1d, Patrícia Ferreira Ribeiro Delfino1e, Rafael Mathias Antonioli1f , Rogério Agenor de Araújo1,3g and Marcelo José Barbosa Silva2h
1Center for Cancer Prevention and Research, Uberlandia Cancer Hospital, Av Amazonas nº 1996, Umuarama, Uberlândia, Minas Gerais, MG 38405-302, Brazil
2Laboratory of Tumor Biomarkers and Osteoimmunology, Institute of Biomedical Sciences, Federal University of Uberlandia, Av Pará nº 1720, Bloco 6T, room 07, Umuarama, Uberlândia, Minas Gerais, MG 38405-320, Brazil
3Medical Faculty, Federal University of Uberlandia, Av Pará nº 1720, Bloco 2U, Umuarama, Uberlândia, Minas Gerais, MG 38400-902, Brazil
ahttps://orcid.org/0000-0002-9381-4913
bhttps://orcid.org/0000-0002-1307-9104
chttps://orcid.org/0000-0002-0955-8559
dhttps://orcid.org/0000-0002-2734-8352
ehttps://orcid.org/0000-0002-2196-9318
fhttps://orcid.org/0000-0003-3886-1562
ghttps://orcid.org/0000-0003-4653-6786
hhttps://orcid.org/0000-0002-5807-4286
Abstract
Background: The luminal subtype accounts for ~70% of newly diagnosed breast cancer patients. Although it has a better prognosis, approximately 30% of them develop a late relapse. Identifying those patients is of interest to improve treatment decisions.
Methods: A retrospective observational, single-centre study based on data from medical records of 572 non-metastatic (I–III) invasive ductal breast carcinoma patients, 448 with luminal tumours and 124 with triple-negative tumours. Kaplan–Meier, Cox regression and time-dependent Cox regression were carried out to obtain the prognosis value of risk factors.
Results: During a median observation of 5.5 years, 105 distant metastasis events and 105 all-cause deaths were observed. In addition to known clinicopathological factors (i.e., age, tumour size and lymph node metastasis), the high semi-quantitative expression of both hormone receptors was associated with distant metastasis-free survival (DMFS) (adjusted hazard ratio (HaR): 0.524 (0.316–0.867), p = 0.012) and overall survival (OS) (adjusted HaR: 0.486 (0.286–0.827), p = 0.008). The stratified analysis made it possible to identify risk modification factors. Subsequent stratification by histological grade, Ki-67 and semi-quantitative PR expression or, mainly, the composite semi-quantitative expression of hormone receptors (cHR) enabled the identification of luminal breast cancer patients of adjuvant schema at higher risk for metastasis and death. However, initial analyses including patients of neoadjuvant therapy pointed to a path of subsequent stratification by cHR and histological grade, also enabling grouping of luminal breast cancer patients with similar prognosis for DMFS (cHR ≤ 4+ G2 or G3 versus triple-negative, adjusted HaR: 0.703 (0.415–1.189), p = 0.189) and OS (cHR ≤4+ G2 or G3 versus triple-negative, adjusted HaR: 0.662 (0.403–1.088), p = 0.104).
Conclusion: The semi-quantitative expression of both cHR, Ki-67 proliferation index and histological grade can identify luminal breast cancer patients at greater risk of developing metastasis and death when combined in a hierarchical fashion, and could be useful for a better prognosis stratification in services from low- and middle-income countries.
Keywords: breast neoplasms; oestrogen receptor alpha; Ki-67 antigen; neoplasm grading; progesterone receptors.
Correspondence to: Felipe Andrés Cordero da Luz
Email: felipecorderodaluz@gmail.com
Published: 04/05/2022
Received: 24/12/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Worldwide, breast cancer is responsible for the majority of cancer deaths in women [1], mainly in the recurrence of the development of distant metastasis [2]. However, breast cancer is a highly heterogeneous disease, being classified as luminal (oestrogen receptor (ER)/ progesterone receptor (PR)-positive, HER2-negative), HER2-enriched (HER2-positive) or triple-negative (negative to ER, PR and HER2) [3].
About 70% of the patients are of the luminal subtype [3, 4]. Although the luminal subtype has a better prognosis than HER2-enriched and triple-negative cancers [5, 6], 25%–30% of them develop resistance to standard endocrine therapy and develop distant relapse in a late pattern [7, 8]. Thus, only the expression of hormone receptors is not sufficient for identifying patients at higher risk of relapse.
Prognostic improvements were further obtained by the use of the proliferation index by Ki-67 [9], the expression level of the progesterone receptor [10] and the histological grade classification by the Nottingham Grading System [11, 12]. However, the current gold standard for identifying luminal patients (ER/PR-positive, HER2-negative) at high risk of developing distant recurrences are genomic tests such as MammaPrint [13–18], Prosigna (Pam50) [17–20], EndoPredict [17, 18, 21, 22] and, especially, Oncotype Dx [17, 18, 23–27]. Despite having positive cost-effectiveness when considering the benefit in quality of life [28–31], the high cost of these tests makes their access unfeasible for a great majority of patients, mainly in low- and middle-income countries [30, 31], hence the importance of using more accessible risk ratings. Nonetheless, the usually evaluated factors in the pathological routine for the diagnosis of patients with breast cancer have a good correlation with such genomic tests [32–37]. But it is necessary to assess these factors together to classify as low- or high-risk diseases.
There are currently two consolidated important classification systems based on histopathological and molecular (immunohistochemistry) factors, but with different approaches. The system by the American Joint Committee on Cancer (AJCC) aims at staging classification by grouping patients with similar overall survival [25], while the St Gallen system aims to group patients with similar risks of developing distant relapses [17, 38]. Even though incorporating almost the same histopathological and immunohistochemical factors in both systems, there are certain discrepancies in the way they are incorporated, such as the qualitative expression of hormone receptors in AJCC [25] and semi-quantitative expression of PR in St Gallen [17, 38]. Possible explanations are the effect of these factors on the analysed outcome or the way to classify them. Another is the interaction of these factors with each other, in which both systems cannot integrate more solidly [17, 25].
This retrospective study aims to assess whether immunohistochemical markers and degree of differentiation can be hierarchically integrated to identify patients with luminal breast cancer at high risk of developing distant metastasis and whether this classification also has a prognosis value for overall survival.
Methods
Study design
A retrospective observational study analysing data collected from the medical records of patients with breast cancer treated at the oncology sector of the Federal University of Uberlandia between January 1999 and December 2019.
Ethical aspects
This study was approved by the Human Research Ethics Committee (protocol number 803.826/14) of the local Institution and followed all the ethical principles of the Declaration of Helsinki and its subsequent amendments or comparable ethical standards. The informed consent form was waived according to the type of study carried out.
Classifications and outcomes
Based on the anatomopathological examination results, and not on the medical notes, all patients were reclassified in their pathological TNM stage according to the Seventh Edition of the AJCC [25]. The highest classification of tumour size (T) and lymph node metastasis (N) between clinical and pathological staging was selected for prognosis factor analysis.
Tumours were classified as luminal when there was a lack of HER2 superexpression/amplification (0/1+ by immunohistochemistry (IHC) and/or lack of ERBB2 amplification by in situ hybridisation technique), and both hormone receptors (ER and/or PR) were expressed in less than 1% of tumour cells. Tumours were classified as triple-negative whenever there was a lack of HER2 superexpression/amplification (0/1+ by IHC and/or lack of ERBB2 amplification by in situ hybridisation technique), and less than 1% of tumour cells expressed both hormone receptors (ER and/or PR) [4].
Patients’ ages were classified by two cut-off values according to the analysed outcome: ≥70 for overall survival due to shorter life expectancy and higher risk of all-cause death receiving fewer treatments [39, 40], and ≤40 for distant metastasis due to increased risk of relapses in younger patients, especially in luminal breast cancer patients [41].
The systemic and radiotherapy treatments were considered adequate whenever patients received treatment as indicated by the current guidelines [10, 17, 42, 43]. Patients were classified as having received or not having received treatment if there was correct adherence or not to the treatment protocols, respectively, as in a previous study [44].
Survival analysis considered the time from diagnosis to censoring or development of the event of interest. As primary and secondary outcomes, the development of distant metastases and general deaths, respectively, were analysed.
Histological and immunohistochemistry methods
Histological grade was evaluated by haematoxylin and eosin stain of histological slides and classification was carried out in accordance with the Nottingham system [12, 45].
Immunohistochemical data were retrieved from IHC reports. IHCs were prospectively carried out at the local laboratory of the institution, according to the good practice guidelines preserved through time [4, 46]. Detection and revealing were carried out by an avidin–biotin–peroxidase system.
According to standard guidelines of the pathology laboratory at the cited institution, immunological and histological analyses were carried out by one pathologist and independently confirmed by another pathologist.
Semi-quantitative expression classification of hormone receptors
The majority of the patients had their hormone receptor (HR) expression reported in the cross-system by Sannino and Shousha [47]. This is an ordinal classification system for hormone receptor expression based on the percentage of tumour cells expressing the marker and the intensity of the label. For standardisation, the few reports in intensity and/or percentage of expressing tumour cells were transformed to this cross system, as available in the literature [46, 47].
Tumours were classified according hormonal receptor positivity as 1+ whenever ≥1% and ≤10% of tumours cells expressed the receptor with low intensity; 3+ whenever 60%–90% of tumours cells expressed the receptor with moderate/strong intensity; and 4+ whenever >90% of the tumour cells expressed the receptor with strong intensity. Tumours with an expression greater than 1+ and less than 3+ are considered undefined as it is a very broad spectrum [46, 47]. Therefore, these patients were not included in the initial analyses.
Posteriorly, patients were also reclassified by the sum of both HR expression patterns in a system from 1 to 8 points. As no consensus has been established for 2+ classification, patients in this situation were included only if the other HR had an expression pattern of 4+, being classified as 5, or 1+, being classified as 3, or 0, being classified as 2. The 2+ classification was only considered if it was accompanied by the percentage and labelling of both receptors to enable the sum of their semi-quantitative expressions. The expression of both hormone receptors, and their composite (sum), was evaluated as continuous variables.
Eligibility criteria
Female patients with invasive ductal carcinoma of no special type histology were included. Patients were excluded by the following criteria: lobular histology or presence of special components (n = 713); exclusive in situ disease (n = 104); synchronic metastatic disease (considering diagnosis within the first 6 months) (n = 174); missing histopathological data (histological grade), lysed/destroyed tumour, incomplete immunohistochemistry (absence of ER, RP, HER2, or Ki67) and/or indeterminate HER2 (2+ with indeterminate/without hybridisation method) (n = 411); failure to perform surgery (n = 6); neoadjuvant radiotherapy (n = 6); bilateral cancer (n = 2); more than one primary cancer (n = 4); follow-up time less than 180 days (6 months) from diagnosis to death/censoring to avoid non-diagnosed synchronous metastasis (n = 19); and HER2 overexpression/amplification (n = 128).
From a total of 2,186 records, 1,567 were excluded based on the aforementioned criteria. After exclusion criteria were applied, 619 patients with non-metastatic, invasive ductal carcinoma of no special type histology of luminal or triple-negative subtype with complete clinicopathological reports were included in the study.
Statistical analysis
Distributions were analysed using the Kolmogorov–Smirnov test. Continuous variables with normal distribution were described as mean (± standard deviation) and non-parametric variables as median (minimum–maximum); categorical variables were described as frequencies.
The association between interdependent categorical factors was evaluated using Pearson’s χ2 test. The association was considered positive (direct) when the adjusted standardised residuals had a value >(+2.0) and considered negative (indirect/inverse) when the value was <(–2.0).
To assess the degree of agreement in the classification of patients between two different approaches, Cohen’s Kappa test was used.
To assess the bivariate correlation in the presence of an ordinal variable, Spearman’s correlation test was used.
To determine the independent prognosis value of the variables, the Cox proportional hazards regression model was used. The proportionality of categorical variables was assessed using the Kaplan–Meier (KM) estimator curve associated with the Log-Rank test.
The proportionality, and linearity, of the risk of continuous variables, was tested by categorising them into defined percentiles. Risk proportionality was assumed as long as and when there were constant risks, evaluated by the KM estimating curve associated with the Log-Rank test. Associated with this, the correlation between the partial residuals generated by the univariable Cox regression with the observation time for each outcome was evaluated; time dependence was assumed in the presence of correlation between these variables and visually analysed by a scatterplot.
In violating the prerequisite of proportionality of risks, time-dependent Cox regression models were used. For categorical variables, observation times were categorised as the time of inflection (crossing) between the curves, and the T_COV variable with interaction (*) with this categorisation was used. Univariable analysis of prognosis factors in a time-dependent context was carried out in the presence of the T_COV* variable.
The Cox regression model with the maximum prognosis value for the analysed cohort was obtained using a two-block analysis. In the first block, candidate factors were included and the Stepwise Forward Wald method was used with an entry p-value equal to 0.10 and output p-value equal to 0.05 to reduce covariate collapsibility [48] and overfitted models; in the second block, the forced entry method of known prognosis factors, like T, N and age, and treatments was used, when pertinent. A second model was built inserting all variables with a p-value < 0.05 in univariable analysis, or with a known theoretical value, to correct any possible overfitting. In risk stratification analyses, the models were always adjusted by T and N for distant metastasis-free survival (DMFS), and T, N and age for overall survival (OS). The simple contrast was used to establish the reference level for categorical variables; the repeated contrast was used to carry out clustering of levels with similar prognosis within a variable.
The aforementioned statistical analyses were carried out with IBM SPSS v25.0.
Using Jamovi v1.6.5.0 software, continuous variables were categorised according to optimal cut-off points obtained by the system after survival analysis of continuous predictors.
A p-value < 0.05 was considered significant for all the aforementioned analyses.
Results
Analysis of risk factors in patients with luminal breast cancer
Of the 619 patients included in the study, 495 and 124 were identified as having luminal and triple-negative breast cancer, respectively. In a median observation period of 64.9 months, 112 events of distant metastasis and 112 deaths were observed. Detailed data are described in Table 1.
To identify the risk factors associated with the development of distant metastases and death in patients with luminal breast cancer, analyses only in this subgroup were carried out.
Survival analyses for continuous variables were carried out for Ki-67 and the quantitative expression of the hormone receptors (HRs) and the optimal cut-off points were identified. For Ki-67, the survival analysis showed no association with DMFS (hazard ratio (HaR): 1.01 (1.00–1.02), p = 0.116). But for OS, an increment of 1% of tumour cells expressing Ki-67 increased by 1% the odds of death (HaR: 1.01 (1.00–1.02), p = 0.009). The optimal cut-offs retrieved by the analysis were >11 and >12 for DMFS and OS, respectively.
Posteriorly, it was tested whether there is a prognosis value of the semi-quantitative expression of HRs and what the optimal cut-off points are. However, 41 patients were classified in category 2+ of the ordinal (cross) system, which has a very broad expression (10%–60% of expressing cells) and generates inconsistencies for semi-quantitative reclassification. Furthermore, nine patients had no quantitative expression either in percentage or in the ordinal system. For this reason, these 50 patients were not included in further analyses. Thus, only 445 patients with luminal tumours were included in subsequent analyses.
For DMFS, the PR semi-quantitative expression (HaR: 0.83 (0.70–0.99), p = 0.035) was significant, meaning a 17% risk decrease by an increment of 1+. The composite semi-quantitative expression of hormone receptors (cHR) showed only a trend for OS (HaR: 0.90 (0.80–1.01), p = 0.064), meaning a 10% risk decrease by an increment of 1 point. The optimal cut-offs retrieved by the analysis were >1 and >4 for PR and cHR, respectively.
For OS, the PR semi-quantitative expression (HaR: 0.81 (0.68–0.96), p = 0.018) and cHR (HaR: 0.89 (0.79–1.00), p = 0.046) were significant, meaning a 19% and 11% risk decrease by an increment of 1 point by each system, respectively. The optimal cut-offs retrieved by the analysis were >1 and >4 for PR and cHR, respectively.
Although with a moderately strong correlation (Spearman’s rho: 0.398, p < 0.005) and association (Pearson’s χ2: 176.768, p < 0.005) between ER and PR, the semi-quantitative expression of ER was not significant for any outcome.
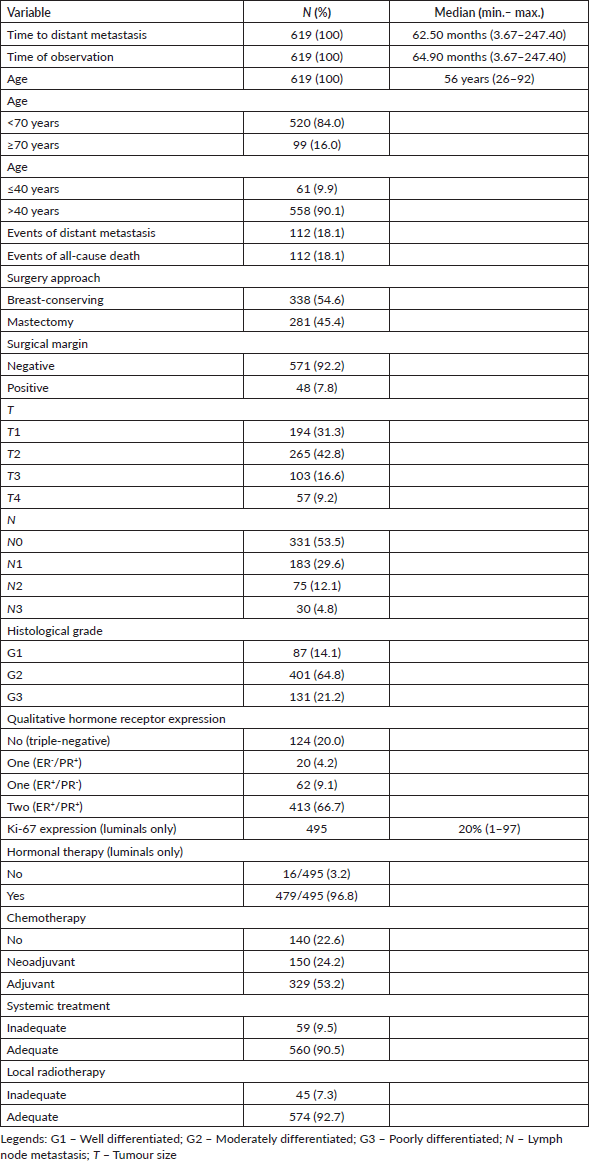
Table 1. Clinical data of included patients in initial analysis (n = 619).
As the results were obtained through the analysis of variables that can violate the assumption of risk proportionality, they were categorised according to the cut-off points obtained in the previous analysis, and proportionality was tested using a KM curve and Log-Rank test. Cut-off points >12, >1 and >4 were selected for Ki-67, PR and cHR, respectively, as a function of significance in the results of previous analyses.
The cHR cut-off enabled the inclusion of three additional patients due to the single expression of ER on their tumours, totalising 448 luminal patients included in further analysis. The clinical data of the 572 patients, 448 with luminal tumours and 124 with triple-negative tumours, for further analysis from this point on are presented in Table 2.
Overall, the categorisation of Ki-67, the semi-quantitative expression of PR and the composite of both hormone receptors showed significance, or were close, for both outcomes (Figures 1–3).
The qualitative expression of hormone receptors and the histological grade also have prognosis values. Therefore, they were evaluated by the KM method. Unlike the semi-quantitative expression, the qualitative expression of hormone receptors was only significant for OS (Figure 4). The histological grade also showed significance, or was close, for both outcomes (Figure 5).
Differently from pathological factors (T and N – data not displayed), the risks remain proportional during the first 60 months (5 years), with changes at later times and intersections being observed close to 120 months (10 years) for some factors (Figures 1–5). Therefore, time-dependent analysis was carried out using an interaction term between the categorised times of observation and the variables of interest.
For DMFS, only the semi-quantitative expressions of PR or cHR resulted in being independent after correction by other factors by stepwise and insert methods, respectively (Table 3).
For OS, the stepwise model included both Ki-67 and qualitative expression of HR as significant (Table 4). Due to strong to very strong correlations between semi-quantitative PR expression and cHR (Spearman’s rho: 0.802, p < 0.005); semi-quantitative PR expression and qualitative HR expression (Spearman’s rho:0.553, p < 0.005); and between cHR and qualitative HR expression (Spearman’s rho: 0.667, p < 0.005), the second model included the factor with the lowest p-value (cHR), which resulted in being significant after covariation (Table 4); the analysis including semi-quantitative PR expression showed significance after covariation (adjusted HaR: 0.465 (0.276–0.781), p = 0.004) and Ki-67 expression (adjusted HaR: 1.803 (1.012–3.210), p = 0.045).
Table 2. Clinical data of included patients in most analysis (n = 572).
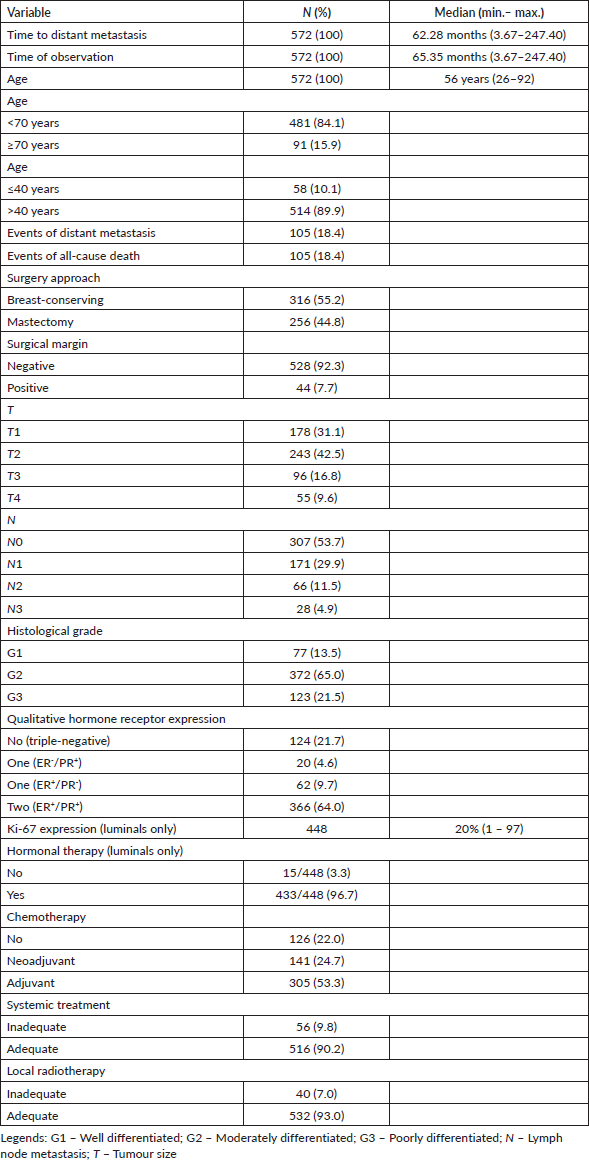
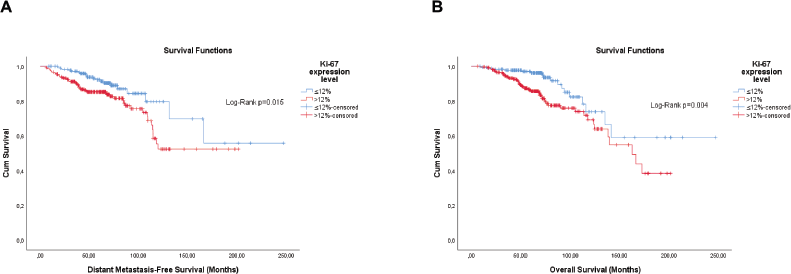
Figure 1. Cumulative survival curves by the KM estimator according to the Ki-67 expression level in luminal breast cancer patients (n = 448). (a): DMFS. (b): OS.
Stratified analysis allowing the identification of risk modification factors
Previous analyses resulted in the inclusion of different factors according to outcomes. However, histological grade and Ki-67 proliferative index are known risk factors in patients with luminal breast cancer. Aiming at a greater segregation of risks, stratified (hierarchical) analyses were carried out to study the effect of modification by other variables. As the semi-quantitative expressions of PR and cHR were independent variables for both outcomes, the hierarchy started with them.
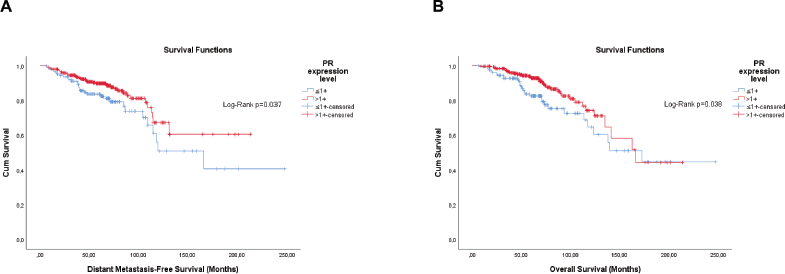
Figure 2. Cumulative survival curves by the KM estimator according to the semi-quantitative expression level of the PR in luminal breast cancer patients (n = 448). (a): DMFS. (b): OS. Legend: PR – Progesterone receptor.
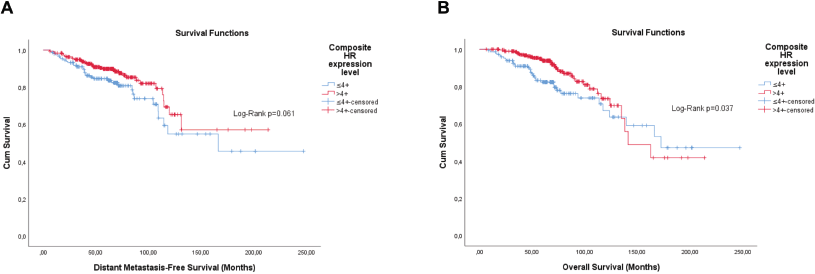
Figure 3. Cumulative survival curves by the KM estimator according to the cHR in luminal breast cancer patients (n = 448). (a): DMFS. (b): OS. Legend: cHR – Composite semi-quantitative expression of hormone receptors.
Starting with the semi-quantitative PR expression level, both Ki-67 and histological grade showed some stratification only in patients with tumours classified as ≤1+. In preliminary analyses, G2 and G3 showed higher overlap for both outcomes. Therefore, these categories were grouped. The Ki-67 level showed significance for both DMFS (Log-Rank; p = 0.003) and OS (Log-Rank; p = 0.007), and histological grade for both DMFS (Log-Rank; p = 0.022) and OS (Log-Rank; p = 0.012) as well. As no interaction was observed (p > 0.05), conventional Cox regression was carried out with the stepwise method. The histological grade (G2/G3 versus G1) resulted in being independent for OS (adjusted HaR: 7.604 (1.007–57.405), p = 0.049), but Ki-67 (>12% versus ≤12%) for DMFS (adjusted HaR: 4.263 (1.331–13.652), p = 0.015).
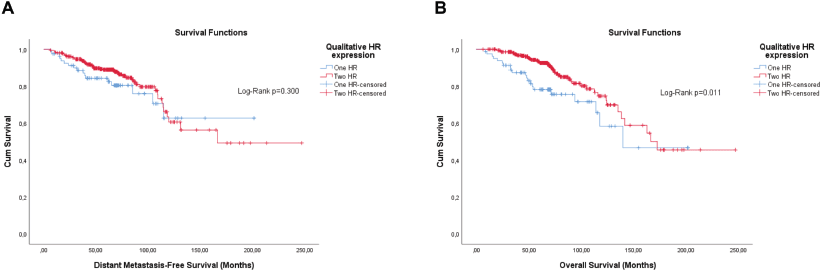
Figure 4. Cumulative survival curves by the KM estimator according to the qualitative expression of hormone receptors in luminal breast cancer patients (n = 448). (a): DMFS. (b): OS.
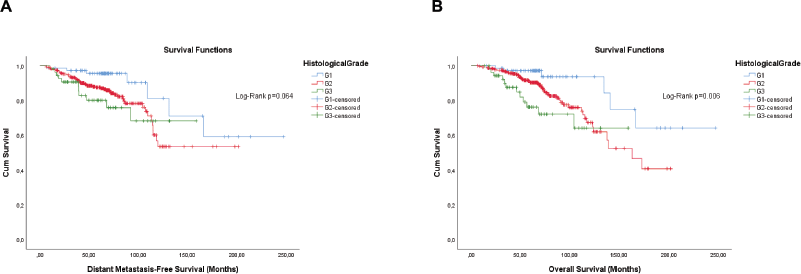
Figure 5. Cumulative survival curves by the KM estimator according to the histological grade in luminal breast cancer patients (n = 448). (a): DMFS. (b): OS. Legend: G1 – Well differentiated; G2 – Moderately differentiated; G3 – Poorly differentiated.
Starting with cHR, both Ki-67 and histological grade showed some stratification only in patients with tumours classified as ≤4+. In preliminary analyses, G2 and G3 showed higher overlap for both outcomes. Therefore, these categories have been grouped. The Ki-67 level showed significance for both DMFS (Log-Rank; p = 0.020) and OS (Log-Rank; p = 0.020), and histological grade for both DMFS (Log-Rank; p = 0.047) and OS (Log-Rank; p = 0.017) as well. As no interaction was observed (p > 0.05), conventional Cox regression was carried out with the stepwise method. The histological grade (G2/G3 versus G1) resulted in being independent for both DMFS (adjusted HaR: 4.506 (1.206–16.827), p = 0.025) and OS (adjusted HaR: 7.601 (1.655 – 34.914), p = 0.009).
Table 3. Univariable and multivariable time-dependent Cox analyses of factors associated with distant metastasis in luminal breast cancer patients (n = 448).
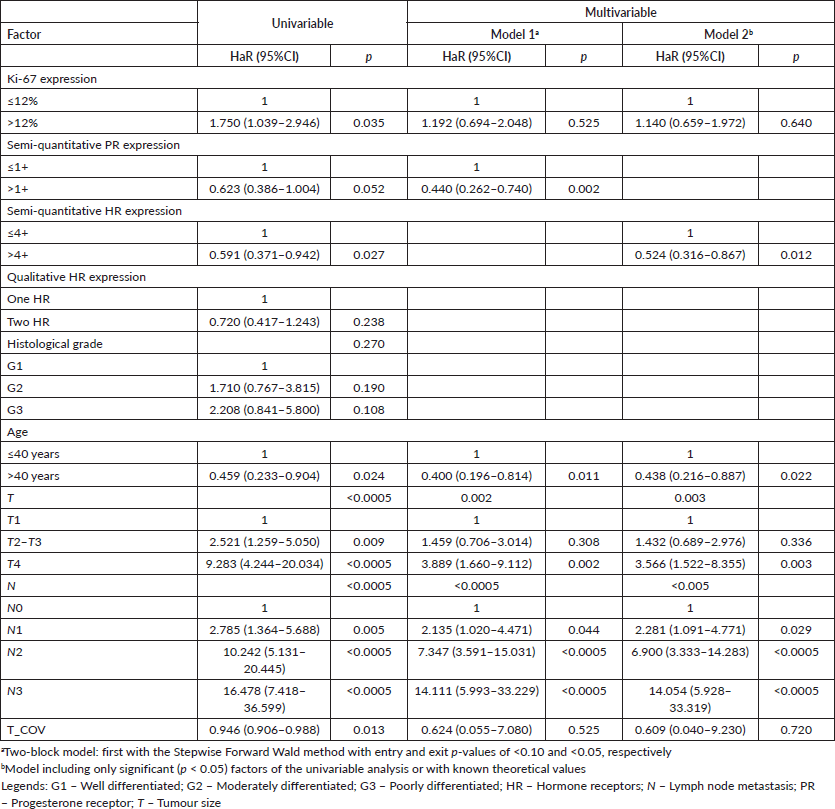
Table 4. Univariable and multivariable time-dependent Cox analyses of factors associated with all-cause death in luminal breast cancer patients (n = 448).
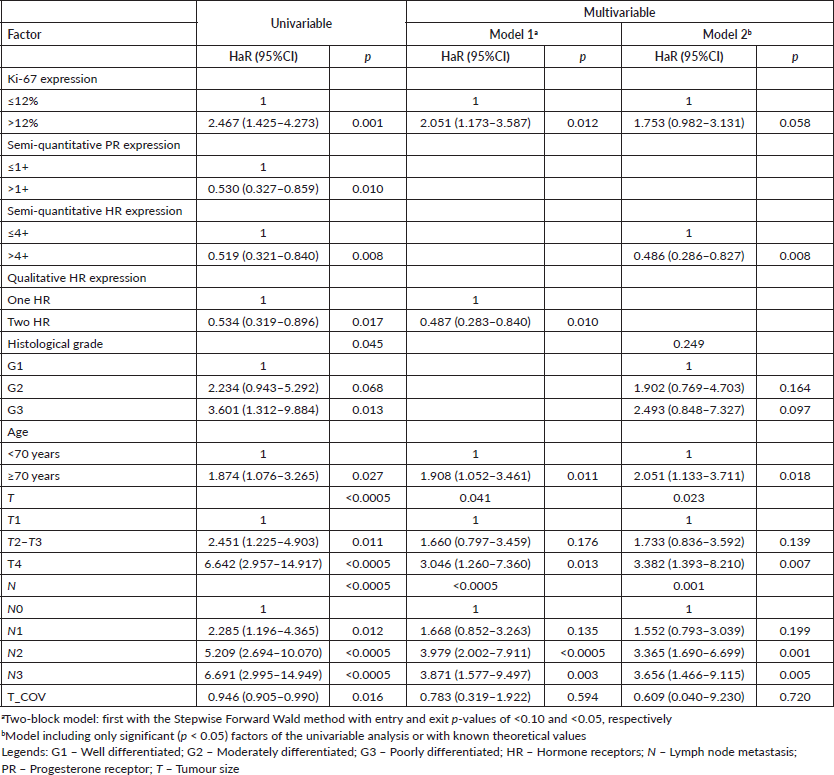
According to the first results, cHR showed consistence as an independent factor for both OS and DMFS. Thus, it is possible to conclude that it would be the best independent risk factor to start hierarchisation. Thus, it was further analysed whether Ki-67 could result in further risk discrimination in these patients, but no risk stratification was obtained. After checking for proportionality assumption by KM curves, time-dependent Cox regression showed that the first two categories (cHR > 4+ and cHR ≤4+ G1) have similar prognosis values for both DMFS (adjusted p=0.454) and OS (adjusted p = 0.290), thus they were grouped in a new two-level variable (cHR > 4+/ cHR ≤4+ G1 and cHR ≤4+ G2/G3).
Because histological grade is a known risk factor for luminal breast cancer patients, another hierarchical risk stratification approach was carried out starting with this variable. After checking for proportionality assumption by KM curves, time-dependent Cox regression showed that both semi-quantitative PR expression (adjusted HaR: 0.341 (0.182–0.639), p = 0.001) and cHR (adjusted HaR: 0.328 (0.180–0.597), p < 0.0005) are independent factors for DMFS, but mutually exclusive, and only for G2. Similarly, time-dependent Cox regression showed, again, that both semi-quantitative PR expression (adjusted HaR: 0.327 (0.174–0.614), p = 0.001) and cHR (adjusted HaR: 0.385 (0.201–0.737), p = 0.004) are independent factors for DMFS, but mutually exclusive, and only for G2. No further stratification was obtained by Ki-67.
By time-dependent Cox multivariable regression, it was possible to observe that in both classifications the two first levels have similar DMFS and OS, and the last two levels have similar DMFS and OS, but different from the first two (data not displayed). Thus, grouping was possible in two-level categories for semi-quantitative PR expression (G1/G2 PR>1+ and G2 PR ≤1+/G3) and cHR (G1/G2 cHR>4+ and G2 cHR ≤4+/G3).
The last approach was carried out starting with Ki-67 levels. For Ki-67>12%, both semi-quantitative PR expression (adjusted HaR: 0.326 (0.179–0.594), p < 0.0005) and cHR (adjusted HaR: 0.414 (0.229–0.823), p = 0.004) are significant for DMFS by time-dependent Cox regression. Again, for Ki-67>12%, both semi-quantitative PR expression (adjusted HaR: 0.387 (0.215–0.700), p = 0.002] and cHR (adjusted HaR: 0.439 (0.234–0.823), p = 0.010) are significant for OS by time-dependent Cox regression. No risk stratification was produced in Ki-67≤12% tumours or by histological grade in further analysis. By time-dependent Cox regression models, it was possible to observe similar prognosis factors by the first two levels of both classifications for DMFS and OS, enabling further clustering.
Regarding the qualitative expression of hormone receptors, stratified (hierarchical) analyses, whether based on this factor or others, did not show potential to segregate risks, since analysis by KM and Log-Rank test showed very high p-values.
Identification of patients at risk similar to those with triple-negative tumours
Previous analyses have shown stratification in different ways and involving different factors. However, there is high collinearity between some of these factors. The semi-quantitative expression classifications of PR and both cHR similarly rank most patients (Cohen’s Kappa: 0.798, p < 0.005). There is correlation between histological grade and Ki-67 (Spearman’s rho: 0.317, p < 0.005), with a moderate association between histological grade and the Ki-67 cut-off (Pearson’s χ2: 27.244, p < 0.005).
None of the classifications by stratification shows 100% agreement with the other. For this reason, all were analysed. As the subsequent classification by cHR and histological grade followed the order of factors found in the initial analysis, its value in multivariable analysis has always been calculated in further analysis.
Triple-negative cancer patients were added as the third level to each category to identify the best risk stratification. As a comparison, the classification of tumours as luminal A and B was according to the expression of PR and Ki-67 (luminal A: PR≥20% and Ki-67<14%; luminal B: PR<20% and/or Ki-67±14%). As there was 100% agreement between the cut-off points obtained (>12%) and that found in the literature (>14%) (Cohen’s Kappa: 1.000, p < 0.005), the former was maintained. As most medical records did not report the percentage of PR-expressing tumour cells, this cut-off point was replaced by PR>1+. Alternatively, the same classification was carried out but with a modified Ki-67 cut-off to 20% as per the modern guidelines.
The stepwise model retained the hierarchical risk stratification by Ki-67 and PR (Tables 5 and 6). Although the classifications similar to those currently accepted are significant, the classifications previously obtained proved to be superior and capable of identifying patients with luminal breast cancer whose prognosis is similar to that of patients with triple-negative breast cancer (Tables 5 and 6).
Table 5. Univariable and multivariable time-dependent Cox analyses of factors associated with distant metastasis in breast cancer patients (n = 572).
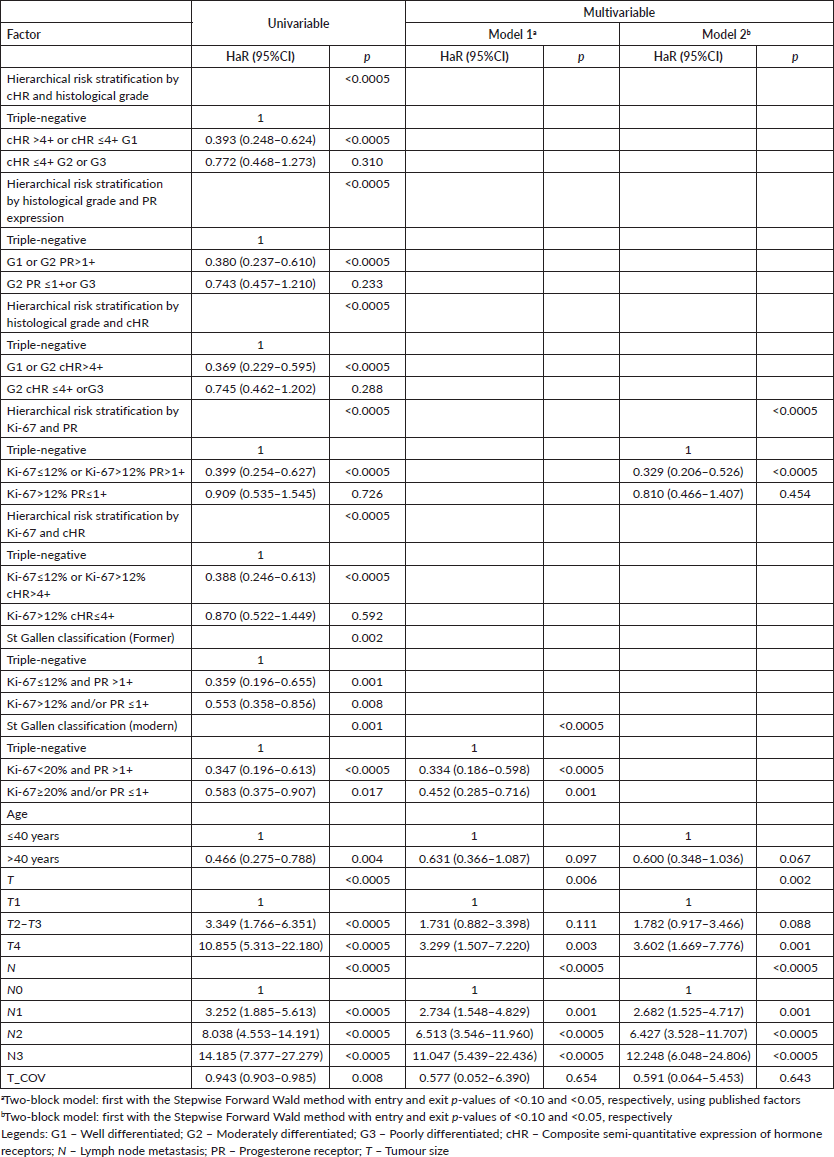
Table 6. Univariable and multivariable time-dependent Cox analyses of factors associated with all-cause death in breast cancer patients (n = 572).
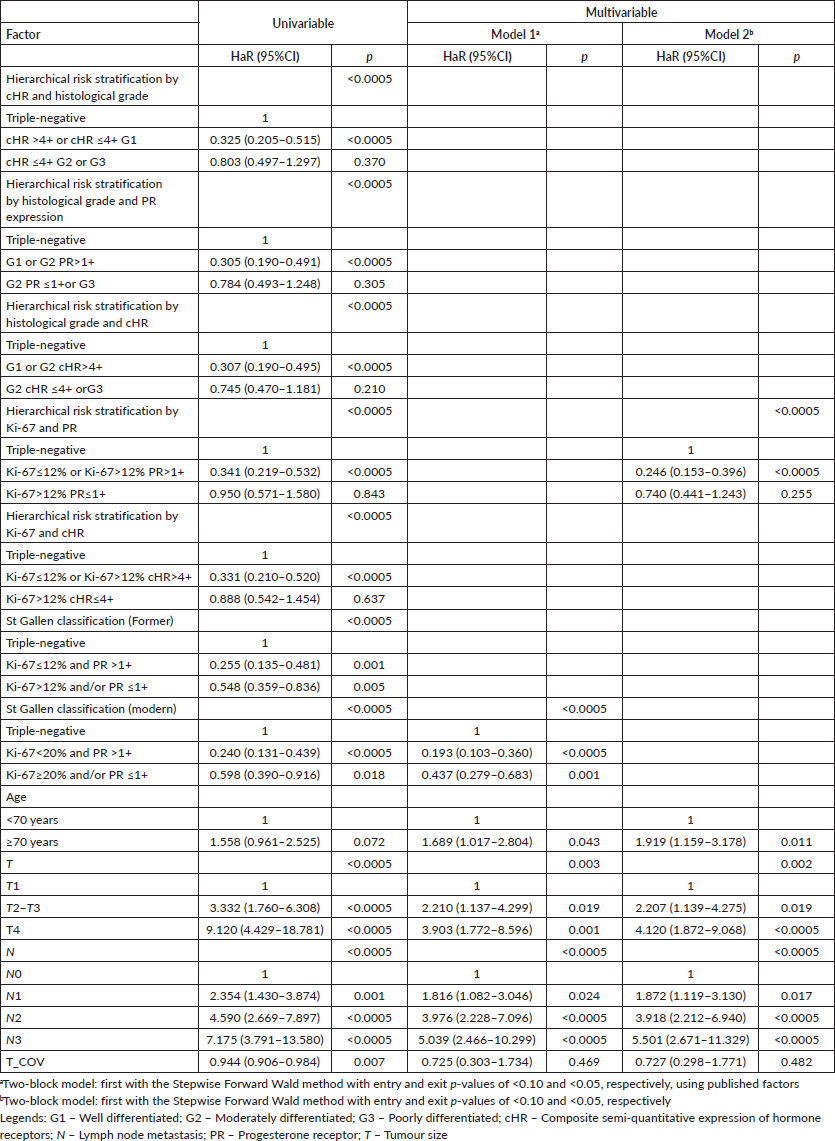
However, the first approach resulted in a good risk stratification and identification of luminal breast tumours patients with prognosis similar to those with triple-negative breast tumours for both DMFS (cHR ≤4+ G2 or G3 versus triple-negative, adjusted HaR: 0.703 (0.415–1.189), p = 0.189) and OS (cHR ≤4+ G2 or G3 versus triple-negative, adjusted HaR: 0.662 (0.403–1.088), p = 0.104).
The second approach also resulted in a good risk stratification and identification of luminal breast tumours patients with prognosis similar to those with triple-negative breast tumours for both DMFS (G2 PR ≤1+or G3 versus triple-negative, adjusted HaR: 0.650 (0.385–1.095), p = 0.106), but with almost a difference for OS (G2 PR ≤1+ or G3 versus triple-negative, adjusted HaR: 0.628 (0.387–1.019), p = 0.059).
Similarly, the third approach resulted in a good risk stratification and identification of luminal breast tumour patients with prognosis similar to those with triple-negative breast tumours for DMFS (G2 cHR ≤4+ or G3 versus triple-negative, adjusted HaR: 0.654 (0.394–1.086), p = 0.101), but not for OS (G2 cHR ≤4+ or G3 versus triple-negative, adjusted HaR: 0.603 (0.373–0.973), p = 0.038).
Finally, the fifth approach resulted in a good risk stratification and identification of luminal breast tumour patients with prognosis similar to those with triple-negative breast tumours for both DMFS (Ki-67>12% cHR≤4+ versus triple-negative, adjusted HaR: 0.691 (0.406–1.174), p = 0.172) and OS (Ki-67>12% cHR≤4+ versus triple-negative, adjusted HaR: 0.695 (0.416–1.161), p = 0.165).
The KM curves of the three fittest approaches are shown in Figures 6–8.
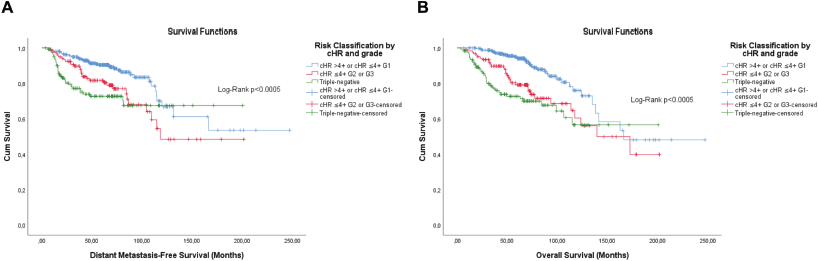
Figure 6. Cumulative survival curves by the KM estimator according to the subsequent risk stratification by cHR and histological grade. (a): DMFS. (b): OS. A total of 572 patients were included. Legend: cHR – Composite semi-quantitative expression of hormone receptors; G1 – Well differentiated; G2 – Moderately differentiated; G3 – Poorly differentiated.
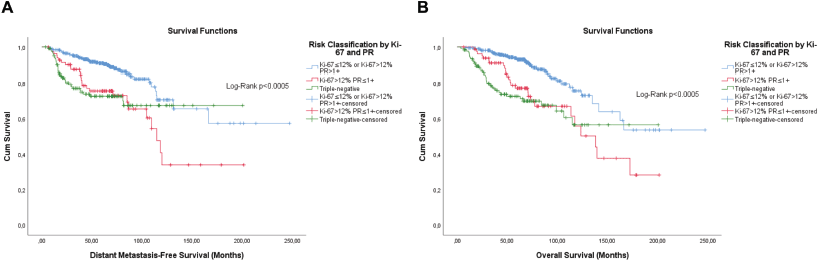
Figure 7. Cumulative survival curves by the KM estimator according to the subsequent risk stratification by Ki-67 and semi-quantitative expression of the progesterone receptor (PR). (a): DMFS. (b): OS. A total of 572 patients were included.
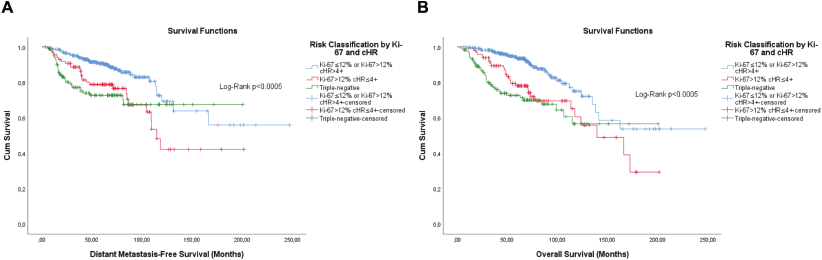
Figure 8. Cumulative survival curves by the KM estimator according to the subsequent risk stratification by Ki-67 and cHR. (a): DMFS. (b): OS. A total of 572 patients were included.
Factors associated with increased odds of metastasis and death in patients of adjuvant schema
Due to the impact that neoadjuvant treatment has on biomarker expression and prognosis, analyses were carried out on the subset of patients who received only adjuvant regimens to test for similar stratifications (n = 358). Unlike the wide variety of possibilities for hierarchies in the total set of luminal breast cancer patients included, stratification based on the histological degree made it possible to discriminate between subgroups with similar prognoses. At the second level of hierarchy, it was possible to observe that Ki-67 promoted prognosis discrimination in patients with grade G1/G2 disease for DMFS (Log-Rank; p = 0.022) and OS (Log-Rank; p = 0.044), being the only significant biomarker in multivariable analysis for DMFS (adjusted HaR: 1.998 (1.019–3.915), p = 0.043), but not significant for OS (adjusted HaR: 1.635 (0.838–3.193), p = 0.150).
Next, we tested whether the semi-quantitative expression of hormone receptors can lead to better discrimination, and a positive response was observed for both PR (DMFS Log-Rank, p = 0.004; OS Log-Rank, p = 0.036) and cHR (DMFS Log-Rank, p = 0.006; OS Log-Rank, p = 0.015), but only in cases with Ki-67 > 12%. It was observed that PR and cHR are independent and superior to DMFS (adjusted HaR: 0.337 (0.155–0.730), p = 0.006) and OS (adjusted HaR: 0.417 (0.189–0.921), p = 0.031), with only a trend for OS (adjusted HaR: 0.498 (0.234–1.058), p = 0.070) and DMFS (adjusted HaR: 0.507 (0.244–1.053), p = 0.069), respectively.
Subsequently, multivariable analyses were carried out to test the possibility of performing clusters. Both risk classification strategies proved to be significant for DMFS and OS. Through repeated contrast, it was possible to observe a clear distinction between the second and third groups for both strategies in the two outcomes tested (data not shown). Therefore, the first two and last two groups were grouped. These classifications have a high, but incomplete, degree of agreement (Cohen’s Kappa: 0.876, p < 0.0005) with each other. Figures 9 and 10 show the survival curves including triple-negative patients also on an adjuvant regimen only.
Adjustments for treatments do not change the prognostic value of risk ratings
Finally, it was analysed whether treatments could be a confounding factor. But first, it was tested in which context chemotherapy results results in a better prognostic for patients with luminal cancer in order to improve the classification of systemic treatment.
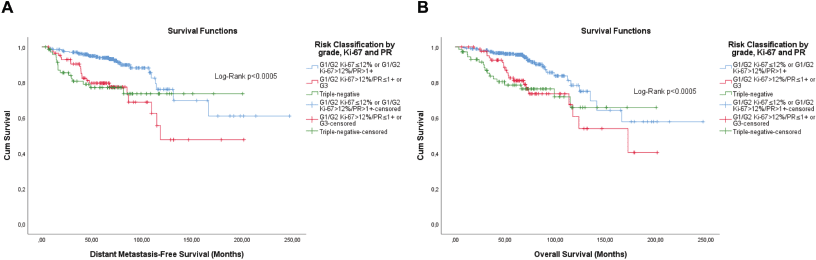
Figure 9. Cumulative survival curves by the KM estimator according to the subsequent risk stratification by grade, Ki-67 and semi-quantitative expression of the progesterone receptor (PR). (a) DMFS. (b): OS. A total of 431 patients of adjuvant schema were included.
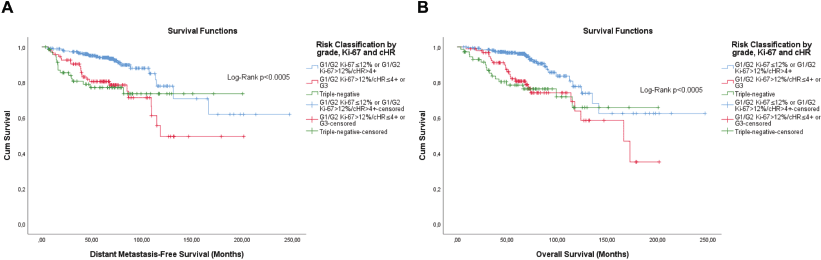
Figure 10. Cumulative survival curves by the KM estimator according to the subsequent risk stratification by grade, Ki-67 and semi-quantitative expression of both hormone receptors (cHR). (a): DMFS. (b): OS. A total of 431 patients of adjuvant schema were included.
Patients with luminal breast cancer from an adjuvant regimen were segregated according to biomarkers and it was evaluated whether chemotherapy implies a better prognosis, since analyses with all patients did not demonstrate a potential benefit of chemotherapy. As it may be an effect modification, the * operation was carried out between pN (N0, N1 and N2/N3) and chemotherapy (No and Yes).
Among the biomarkers, the only one that showed ‘predictive’ value was Ki-67. It was possible to observe that pN is the main factor associated with lower DMFS. It was observed that pN2/N3 is a high-risk factor associated with DMFS (versus pN0, adjusted HaR: 20.778 (6.532–66.092) p < 0.0005) and chemotherapy modifies the effect on pN2/N3 (adjusted HaR: 0.093 (0.010–0.833) p = 0.034), but not on pN1
(p = 0.610). Such an effect modification was also observed regarding OS for pN2/N3 (adjusted HaR: 0.103 (0.014–0.743) p = 0.024), but not for pN1 (p = 0.858).
As chemotherapy was not significant in the models to validate its interaction with lymph node metastasis, subgroup analyses were carried out. In fact, chemotherapy was observed to be the only factor associated with OS (HaR: 0.263 (0.092–0.752) p = 0.013) in patients with pN2/N3 and Ki-67>12% disease, with a trend for DMFS in association to radiation therapy for DMFS (adjusted HaR: 0.346 (0.115 – 1.045) p = 0.060).
Regarding risk classifications, only the last two showed a ‘predictive’ value of benefit from chemotherapy, but only for OS. Patients with pN2/N3 disease and tumors classified as G1/G2 Ki-67>12%/cHR≤4+ or G3 (adjusted HaR: 0.043 (0.002–0.758) p = 0.032), or G1/G2 Ki-67>12%/PR≤1+ or G3 disease (adjusted HaR: 0.039 (0.002–0.725) p = 0.030) showed effect modification by chemotherapy, but again, without chemotherapy as significant in the models. However, a trend of benefit of chemotherapy was observed in the first group only by subgroup analysis according to pN2/N3 disease (HaR: 0.252 (0.060–1.064) p = 0.061).
Therefore, systemic treatment was re-categorised based on the previous results, assigning appropriate treatment classifications to patients whenever they received hormone therapy, and they received chemotherapy if and only if they had pN2/3 disease and Ki-67>12%, which resulted superior to the initial classification, according to the St Gallen guidelines.
Finally, models were adjusted to include adjuvant regimen-only treatments. For this, time from the end of the adjuvant therapies (chemotherapy or radiotherapy) until the event or censorship was considered; patients who developed events during adjuvant therapy (chemotherapy or radiotherapy) were excluded. A total of 419 patients were included in the analyses. Two models using the two-block approach were built. The first model included all risk ratings in the first block; the second model was designed excluding the first block and the risk classification obtained in the first model.
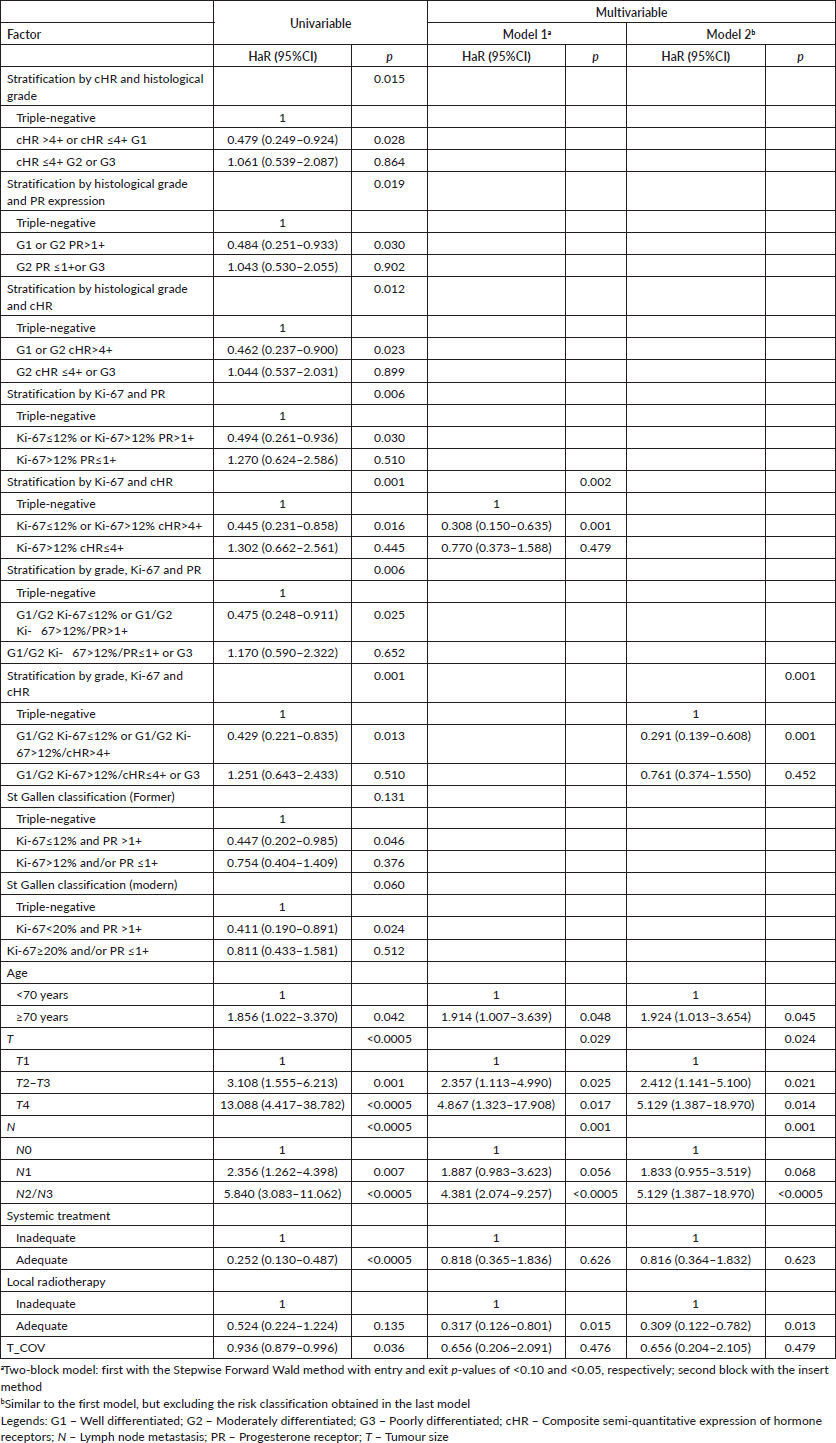
The stratifications with Ki-67 in the first hierarchical level continued to be statistically superior to the others, even compared to those described in the literature, with change from PR, at the second hierarchical level referring to DMFS, to cHR, at the second hierarchical level, and to OS (Tables 7 and 8). Nonetheless, the second model for both DMFS and OS agreed that the sequential risk stratification by histological grade, Ki-67 and cHR as independent factors is superior to the others (Tables 7 and 8).
Risk stratifications involving only histological grade and semi-quantitative expression of hormone receptor(s), regardless of the order, did not result in good identification of patients with luminal cancer whose prognoses are similar to triple-negative. Regarding DMFS, the second category of subsequent stratification by cHR and grade, grade and PR, and grade and cHR showed a better prognosis than triple-negative tumours with p-values of 0.034 (adjusted HaR: 0.415 (0.184–0.937)), 0.064 (adjusted HaR: 0.473 (0.214–1.045)) and 0.050 (adjusted HaR: 0.456 (0.209–0.999)), respectively. Regarding OS, the second category of subsequent stratification by cHR and grade, grade and PR, and grade and cHR showed similar prognosis than triple-negative tumours with p-values of 0.336 (adjusted HaR: 0.704 (0.344–1.440)), 0.374 (adjusted HaR: 0.721 (0.350–1.484)) and 0.333 (adjusted HaR: 0.706 (0.349–1.429)), respectively.
Discussion
The identification of luminal breast cancer cases with a high risk of metastasis development is critical for a therapeutic decision [10, 17]. However, the only prognostic and predictive test currently available with grade 1A evidence for both therapeutic decision and staging is the Oncotype Dx genomic test [49–51], which is expensive.
Studies have shown consecutive risk stratification in luminal cancer cases by Ki-67 [9, 10], with subsequent prognostic gain by segregation according to the semi-quantitative expression of PR [52], but the unification with the histological grade remains a difficulty [10, 17, 38]. Ehinger et al [53] observed that, in patients with luminal ER+ tumours, the semi-quantitative expression of PR and Ki-67 discriminates prognosis only in moderately differentiated (G2) tumours. Furthermore, although other studies show the Ki-67 index as a risk segregation criterion in G2 tumours [12, 54], Liang et al [55] observed similar prognosis between patients with G3 tumours and high Ki-67 expression in G1 and G2 tumours regarding recurrences. We were able to incorporate these three factors but only in patients of adjuvant regimen, with consecutive hierarchical levels by histological grade, Ki-67 and, finally, the semi-quantitative expression of both hormone receptors, or just of the progesterone receptor. Even so, the stratification path that the initial analysis pointed to was another, promoting consecutive stratification by cHR and later by histological grade, showing similar prognosis between G2 and G3 diseases with low expression of both hormone receptors. Notwithstanding, this stratification did not result in a good risk segregation for DMFS in patients of adjuvant schema as we observed for consecutive stratification by histological grade, Ki-67 proliferation index and, finally, cHR.
Table 7. Univariable and multivariable time-dependent Cox analyses of factors associated with distant metastasis in breast cancer patients in the presence of adjuvant treatments (n = 419).
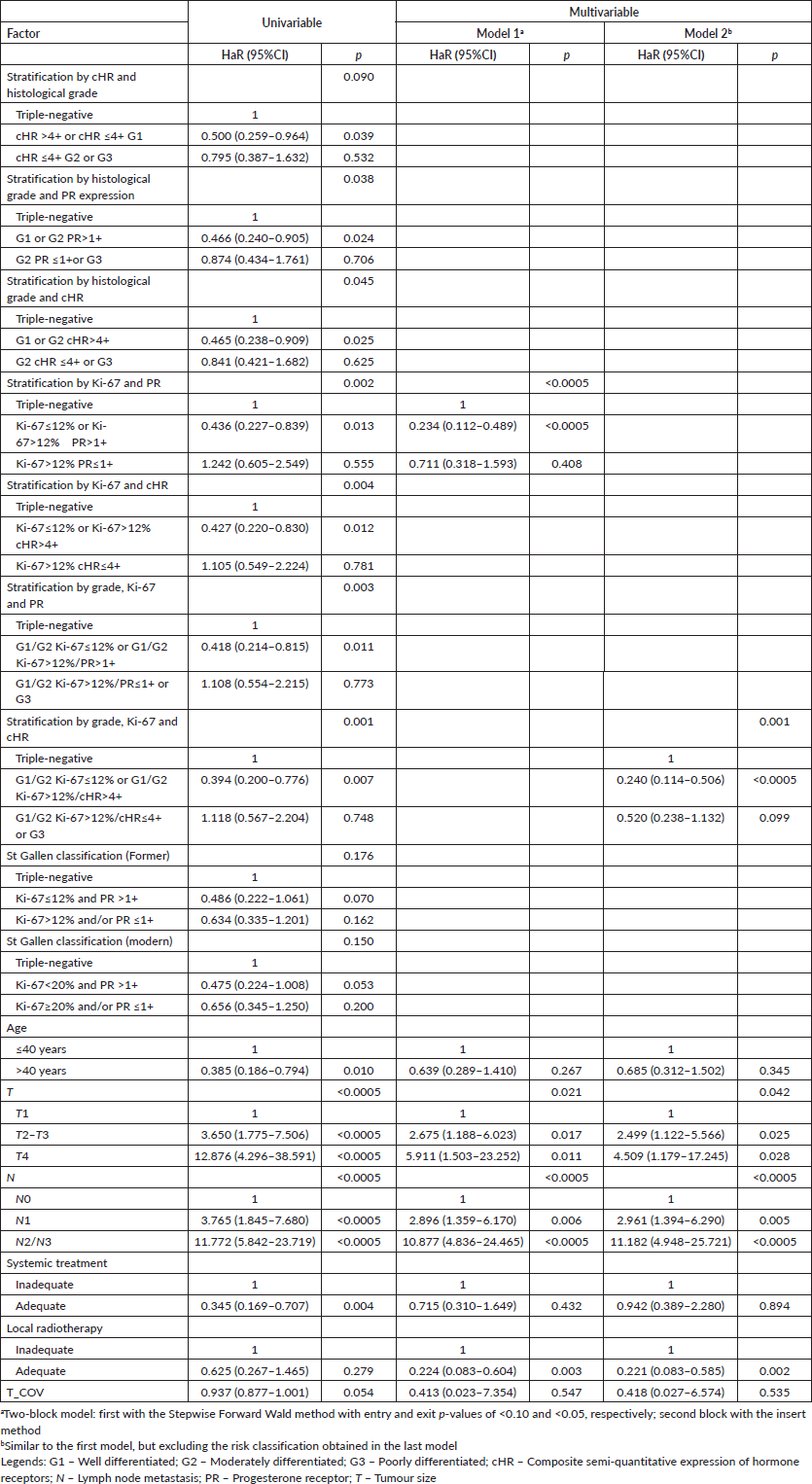
Table 8. Univariable and multivariable time-dependent Cox analyses of factors associated with all-cause death in breast cancer patients in the presence of adjuvant treatments (n = 419).
Interestingly, all classifications obtained in the analysis turned out to be superior to the currently accepted luminal breast cancer classification (luminal A and B) [10, 17]. However, due to the limitation in reporting the expression of hormone receptors, only one approximation was made. In fact, the used PR >1+ category represents tumours with more than 10% expressing cells [46, 47], which is biased while making proper comparisons.
Even though the classification incorporating PR levels proved to be interesting, the classifications involving cHR respected some theoretical foundations. For example, luminal tumours expressing only the PR are more aggressive, being a factor of poorer prognosis for relapses and death, even when compared to ER+/PR- cases [56–60]. Additionally, a recent meta-analysis demonstrated that low ER-expressing (1%–10%) tumours have an impaired endocrine response [61], a typical characteristic of more aggressive luminal tumours [62]. Thus, classification considering cHR naturally classified tumours with low ER expression and expression of only one hormone receptor as similar, although this approach is not largely validated.
Another important theoretical factor is how the interaction between the receptors takes place. In oestrogen-responsive tumours, PR is expressed as a response to the latter via the ER [63], with subsequent modification of the ER interaction network and expression of different target genes in a feedforward fashion [64]. In line with this, we observed that low ER-expressing tumours are likely to express low PR levels, whereas high ER-expressing tumours express high levels of PR, suggesting that this effect probably occurs in a dose-dependent manner. Thus, the semi-quantitative expression of both HRs could respond for part of the highly intricate biology of breast cancers, as we observed for both studied outcomes, and it respects the fact that the qualitative expression of HR is a more important prognosis factor for OS.
Genetic studies with different panels show segregation of the histological grade into two genetic grades (low and high), which have a higher prognosis value than the histological grade [65]. Interestingly, there is a high degree of agreement between the histological grade G1 and the low genetic grade and the histologic grade G3 with the high genetic grade, but high segregation in the histological grade G2 between these two genetic grades [66, 67]. In fact, the histological grade G2 is the classification with the greatest disagreement among the three [68–70], and some studies suggest that the proliferative index can promote a prognosis distinction precisely in G2 [12, 54].
A study by Sotiriou et al [71] observed that low and high genetic grades can segregate patients with PR+ tumours according to endocrine responsiveness and cell proliferation, whereas the low genetic grade has lower proliferation and greater endocrine responsiveness. We observed that the semi-quantitative expression made efficient segregation of the risk factor, but only in G1/G2 patients with a high Ki-67 proliferative index, which finally defined the luminal patients in two groups, which is in harmony with the study by Arima et al [72], in which they observed that in luminal patients (HER2-) the expression of PR does not add a prognosis factor in patients with low proliferative index by Ki-67, but the high expression of RP (≥20%) partially counterbalances the negative effect of the high proliferative index by Ki-67, as we observed in this study.
The low sample number of patients with G1 and G3 tumours prevented us from evaluating whether these other two factors would cause any segregation of risks. However, Ehinger et al [53] observed that patients with luminal breast cancer (ER+/HER2-) of histological grade G3 have a similar prognosis factor, regardless of whether they are classified as luminal A or B based on the Ki-67 proliferative index and qualitative PR expression. In fact, there is a consensus that high histological grade (G3) is a factor of worse prognosis in luminal A patients [10].
Furthermore, an expression of both hormone receptors, not just PR, correlates with the Oncotype Recurrence Score [35, 37, 73], and has a prognosis value for distant metastases by the IHC4 predictive model [74], which has a similar prognosis value to Oncotype Dx for early metastases (up to 5 years) [74–76]. Thus, there is more than enough scientific support to analyse the semi-quantitative expression of both receptors as a prognosis factor, and it is versatile in allowing both risk segregation for distant metastasis and potential staging for overall survival, unlike the qualitative expression which is a known staging factor for overall survival [25], as we also observed in the initial analyses.
In this study, the histological grade and the Ki-67 proliferative index proved to be mutually exclusive in several analyses. One possibility is the correlation that exists between them [77], because the mitotic index is one of the classification criteria of the Nottingham system [11, 45]. Another possibility is the changes that neoadjuvant chemotherapy causes in the tumour microenvironment and in the selection of subclones with different phenotypes, causing changes in the expression of hormone receptors, mitotic index and Ki-67 [78–83], which may explain why it was possible to incorporate histological grade and Ki-67 only in patients on the adjuvant regimen, but not in the group including those on the neoadjuvant regimen.
Although showing the superiority of prognosis segregation in the incorporation of the three analysed parameters (histological grade, Ki-67 and semi-quantitative expression of hormone receptors), having presented other stratification models is interesting for some situations. For example, the treatment guidelines approved for the public health system in Brazil make the assessment of histological grade and identification of the three important tumour receptors mandatory, but not Ki-67 [84].
An interesting factor related to the study of these biomarkers is the potential benefit of chemotherapy. Although the focus was not the discovery of a predictive classification system for the benefit of chemotherapy, it was possible to observe that Ki-67 is the best predictive factor for this response, but only in patients with pN2/N3 disease. This is in agreement with data in the literature, which show that Ki-67 is the best predictor of response to chemotherapy in patients with luminal breast cancer [85, 86]. Interestingly, we could observe that patients with G1/G2 Ki-67>12%/cHR≤4+ or G3 disease show a modification of effect by chemotherapy when there is the involvement of four or more lymph nodes (pN2/N3) for OS. In fact, there is evidence that patients with tumours of good biological risk (low grade, low proliferation and increased expression of hormone receptors) have little or no benefit from chemotherapy, even in the presence of metastatic lymph nodes, but the contrary is true [62]. Thus, this hierarchical classification can even help in clinical decision-making for treatment.
This study has several limitations, as the long elapsed period, which implies the incorporation of new therapies. Other limitations are the possibility of IHC analysis artefacts [4], which we did not have access to, and the reproducibility and feasibility of different antibody clones [4, 87–90], which were used by the Institution, that could dampen our results. Also, the low number of patients representing certain histopathological and immunohistochemical features, such as G1 and G3 tumours, and tumours with low expressions of hormone receptors and/or only one hormone receptor led to results with very wide confidence intervals due to the low number of events observed in these categories.
Regarding Ki-67, we analysed only one dichotomisation, according to the statistical methodology used. However, it is known that there are multilevel categorisations of Ki-67 that better relate to the development of relapses and response to chemotherapy [17, 38, 62, 85, 86].
In addition, we could not observe the median time in most analyses, being observed practically only for the second category of classification of luminal patients of all reclassifications, but not for the first category or patients with triple-negative tumours, which makes the results on prognosis factors dependent on the survival curves of this cohort.
But the main limitation is the classification of the semi-quantitative expression of hormone receptors. Due to the lack of standardisation of the semi-quantitative expression of these, which has recently been incorporated into the guidelines of the public health system in Brazil [84], we needed to standardise the reports to the ordinal system of crosses by Sannino and Shousha [46, 47]. This leads to the loss of information that more refined systems provide, such as Allred or H-score systems [91, 92]. Despite these limitations, our results do not greatly contrast with those in the literature.
A study with a large cohort and improved immunohistochemical data (percentage and intensity) could show the validity of the presented approach and whether it is possible to integrate histological grade and Ki-67, along with semi-quantitative expression of hormone receptors, to identify patients at high risk of developing distant metastases and death.
Conclusion
The different risk stratifications prove to be advantageous when there are limitations of information obtained by IHC techniques according to clinical practices adopted in regions with few resources, such as in low- and middle-income countries. The fact that risk stratification with better identification of high-risk patients, similar to those with triple-negative cancer, was generated by the incorporation of all the variables included attests to the need to include a detailed analysis of these factors in the routines and guidelines of countries such as Brazil.
List of abbreviations
AJCC: American Joint Committee on Cancer
cHR: Composite semi-quantitative expression of hormone receptors
DMFS: Distant metastasis-free survival
ER: Oestrogen receptor
G1: Well-differentiated histological grade
G2: Moderately differentiated histological grade
G3: Poorly differentiated histological grade
HR: Hormone receptor
HaR: Hazard ratio
IHC: Immunohistochemistry
KM: Kaplan–Meier estimator curve
N: Lymph node metastasis, categorisation by AJCC guidelines
OS: Overall survival
PR: Progesterone receptor
T: Tumour size, categorisation by AJCC guidelines
Conflicts of interest
The authors declared no conflicts of interest.
Funding statement
The authors received no financial support for the research, authorship and/or publication of this article.
Author contributions
FACL conceived the study. FACL, ECM, CPN, LAM and PFRD designed the methodology and collected data. FACL carried out the data analysis. RMF, RAR and MJBS supervised and validated the data collection and analysis. FACL wrote the initial manuscript. All authors reviewed and approved the final draft.
Data availability
The data generated for this study is publicly available at DOI: 10.17632/k4ycfhbmbx.3
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Dillekas H, Rogers MS, and Straume O (2019) Are 90% of deaths from cancer caused by metastases? Cancer Med 8(12) 5574–5576 https://doi.org/10.1002/cam4.2474 PMID: 31397113 PMCID: 6745820
3. Network TCGA (2012) Comprehensive molecular portraits of human breast tumours Nature 490(7418) 61–70 https://doi.org/10.1038/nature11412
4. Hammond ME, Hayes DF, and Dowsett M, et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med 134(7) e48–e72 https://doi.org/10.5858/134.7.e48 PMID: 20586616
5. van Maaren MC, de Munck L, and Strobbe LJA, et al (2019) Ten-year recurrence rates for breast cancer subtypes in the Netherlands: a large population-based study Int J Cancer 144(2) 263–272 https://doi.org/10.1002/ijc.31914
6. Jensen MB, Nielsen TO, and Knoop AS, et al (2018) Mortality and recurrence rates among systemically untreated high risk breast cancer patients included in the DBCG 77 trials Acta Oncol 57(1) 135–140 https://doi.org/10.1080/0284186X.2017.1400181
7. Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials Lancet 365(9472) 1687–1717 https://doi.org/10.1016/S0140-6736(05)66544-0 PMID: 15894097
8. Laible M, Hartmann K, and Gurtler C, et al (2019) Impact of molecular subtypes on the prediction of distant recurrence in estrogen receptor (ER) positive, human epidermal growth factor receptor 2 (HER2) negative breast cancer upon five years of endocrine therapy BMC Cancer 19(1) 694 https://doi.org/10.1186/s12885-019-5890-z PMID: 31307414 PMCID: 6631550
9. Goldhirsch A, Wood WC, and Coates AS, et al (2011) Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011 Ann Oncol 22(8) 1736–1747 https://doi.org/10.1093/annonc/mdr304 PMID: 21709140 PMCID: 3144634
10. Goldhirsch A, Winer EP, and Coates AS, et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013 Ann Oncol 24(9) 2206–2223 https://doi.org/10.1093/annonc/mdt303 PMID: 23917950 PMCID: 3755334
11. Rakha EA, El-Sayed ME, and Lee AHE, et al (2008) Prognostic significance of nottingham histologic grade in invasive breast carcinoma J Clin Oncol 26(19) 3153–3158 https://doi.org/10.1200/JCO.2007.15.5986 PMID: 18490649
12. Rakha EA, Reis-Filho JS, and Baehner F, et al (2010) Breast cancer prognostic classification in the molecular era: the role of histological grade Breast Cancer Res 12(4) 207 https://doi.org/10.1186/bcr2607 PMID: 20804570 PMCID: 2949637
13. van ‘t Veer LJ, Dai H, and van de Vijver MJ, et al (2002) Gene expression profiling predicts clinical outcome of breast cancer Nature 415(6871) 530–536 https://doi.org/10.1038/415530a
14. van de Vijver MJ, He YD, and van’t Veer LJ, et al (2002) A gene-expression signature as a predictor of survival in breast cancer N Engl J Med 347(25) 1999–2009 https://doi.org/10.1056/NEJMoa021967 PMID: 12490681
15. Perou CM, Sorlie T, and Eisen MB, et al (2000) Molecular portraits of human breast tumours Nature 406(6797) 747–752 https://doi.org/10.1038/35021093 PMID: 10963602
16. Cardoso F, van’t Veer LJ, and Bogaerts J, et al (2016) 70-gene signature as an aid to treatment decisions in early-stage breast cancer N Engl J Med 375(8) 717–729 https://doi.org/10.1056/NEJMoa1602253 PMID: 27557300
17. Coates AS, Winer EP, and Goldhirsch A, et al (2015) Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015 Ann Oncol 26(8) 1533–1546 https://doi.org/10.1093/annonc/mdv221 PMID: 25939896 PMCID: 4511219
18. Krop I, Ismaila N, and Andre F, et al (2017) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update J Clin Oncol 35(24) 2838–2847 https://doi.org/10.1200/JCO.2017.74.0472 PMID: 28692382 PMCID: 5846188
19. Parker JS, Mullins M, and Cheang MC, et al (209) Supervised risk predictor of breast cancer based on intrinsic subtypes J Clin Oncol 27(8) 1160–1167 PMID: 19204204 PMCID: 2667820
20. Nielsen TO, Parker JS, and Leung S, et al (2010) A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer Clin Cancer Res 16(21) 5222–5232 https://doi.org/10.1158/1078-0432.CCR-10-1282 PMID: 20837693 PMCID: 2970720
21. Gyanchandani R, Lin Y, and Lin HM, et al (2016) Intratumor heterogeneity affects gene expression profile test prognostic risk stratification in early breast cancer Clin Cancer Res 22(21) 5362–5369 https://doi.org/10.1158/1078-0432.CCR-15-2889 PMID: 27185370 PMCID: 5093028
22. Martin M, Brase JC, and Calvo L, et al (2014) Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2- breast cancer patients: results from the GEICAM 9906 trial Breast Cancer Res 16(2) R38 https://doi.org/10.1186/bcr3642 PMID: 24725534 PMCID: 4076639
23. Albain KS, Barlow WE, and Shak S, et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial Lancet Oncol 11(1) 55–65 https://doi.org/10.1016/S1470-2045(09)70314-6
24. Paik S, Tang G, and Shak S, et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer J Clin Oncol 24(23) 3726–3734 https://doi.org/10.1200/JCO.2005.04.7985 PMID: 16720680
25. Hortobagyi GN, Connolly CE, and D’Orsi CJ, et al (2017) Breast AJCC cancer staging manual eds MB Amin, S Edge, and F Greene, et al 8th edn (New York: Springer) pp 589–636 https://doi.org/10.1007/978-3-319-40618-3_48
26. Sparano JA, Gray RJ, and Makower DF, et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer N Engl J Med 379(2) 111–121 https://doi.org/10.1056/NEJMoa1804710 PMID: 29860917 PMCID: 6172658
27. Sparano JA, Gray RJ, and Ravdin PM, et al (2019) Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer N Engl J Med 380(25) 2395–2405 https://doi.org/10.1056/NEJMoa1904819 PMID: 31157962 PMCID: 6709671
28. Yang M, Rajan S, and Issa AM (2012) Cost effectiveness of gene expression profiling for early stage breast cancer: a decision-analytic model Cancer 118(20) 5163–5170 https://doi.org/10.1002/cncr.27443 PMID: 22359236
29. Chandler Y, Schechter CB, and Jayasekera J, et al (2018) Cost effectiveness of gene expression profile testing in community practice J Clin Oncol 36(6) 554–562 https://doi.org/10.1200/JCO.2017.74.5034 PMID: 29309250 PMCID: 5815401
30. Bacchi CE, Prisco F, and Carvalho FM, et al (2010) Potential economic impact of the 21-gene expression assay on the treatment of breast cancer in Brazil Rev Assoc Med Bras (1992) 56(2) 186–191 https://doi.org/10.1590/S0104-42302010000200017
31. Ozmen V, Cakar B, and Gokmen E, et al (2019) Cost effectiveness of gene expression profiling in patients with early-stage breast cancer in a middle-income country, Turkey: results of a prospective multicenter study Eur J Breast Health 15(3) 183–190 https://doi.org/10.5152/ejbh.2019.4761 PMID: 31312795 PMCID: 6619785
32. Kim HS, Umbricht CB, and Illei PB, et al (2016) Optimizing the use of gene expression profiling in early-stage breast cancer J Clin Oncol 34(36) 4390–4397 https://doi.org/10.1200/JCO.2016.67.7195 PMID: 27998227 PMCID: 5455310
33. Orucevic A, Bell JL, and King M, et al (2019) Nomogram update based on TAILORx clinical trial results – oncotype DX breast cancer recurrence score can be predicted using clinicopathologic data Breast 46 116–125 https://doi.org/10.1016/j.breast.2019.05.006 PMID: 31146185
34. Eaton AA, Pesce CE, and Murphy JO, et al (2017) Estimating the oncotypeDX score: validation of an inexpensive estimation tool Breast Cancer Res Treat 161(3) 435–441 https://doi.org/10.1007/s10549-016-4069-4 PMCID: 5310948
35. Bhargava R, Clark BZ, and Carter GJ, et al (2020) The healthcare value of the Magee Decision Algorithm: use of Magee Equations and mitosis score to safely forgo molecular testing in breast cancer Mod Pathol 33(8) 1563–1570 https://doi.org/10.1038/s41379-020-0521-4 PMID: 32203092 PMCID: 7384988
36. Slembrouck L, Vanden Bempt I, and Wildiers H, et al (2021) Concordance between results of inexpensive statistical models and multigene signatures in patients with ER+/HER2- early breast cancer Mod Pathol 34(7) 1297–1309 https://doi.org/10.1038/s41379-021-00743-8 PMID: 33558657
37. Baskota SU, Dabbs DJ, and Clark BZ, et al (2021) Prosigna(R) breast cancer assay: histopathologic correlation, development, and assessment of size, nodal status, Ki-67 (SiNK) index for breast cancer prognosis Mod Pathol 34(1) 70–76 https://doi.org/10.1038/s41379-020-0643-8
38. Burstein HJ, Curigliano G, and Loibl S, et al (2019) Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019 Ann Oncol 30(10) 1541–1557 https://doi.org/10.1093/annonc/mdz235 PMID: 31373601
39. Wyld L, Reed MWR, and Morgan J, et al (2021) Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life Eur J Cancer 142 48–62 https://doi.org/10.1016/j.ejca.2020.10.015 PMCID: 7789991
40. Wyld L, Reed MWR, and Collins K, et al (2021) Bridging the age gap in breast cancer: cluster randomized trial of the effects of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices Br J Surg 108(5) 499–510 https://doi.org/10.1093/bjs/znab005 PMID: 33760077
41. Lian W, Fu F, and Lin Y, et al (2017) The impact of young age for prognosis by subtype in women with early breast cancer Sci Rep 7(1) 11625 https://doi.org/10.1038/s41598-017-10414-x PMID: 28912475 PMCID: 5599495
42. Smith BD, Bellon JR, and Blitzblau R, et al (2018) Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline Pract Radiat Oncol 8(3) 145–152 https://doi.org/10.1016/j.prro.2018.01.012 PMID: 29545124
43. Budach W, Bolke E, and Kammers K, et al (2015) Adjuvant radiation therapy of regional lymph nodes in breast cancer – a meta-analysis of randomized trials–an update Radiat Oncol 10 258 https://doi.org/10.1186/s13014-015-0568-4
44. Luz FAC, Marinho EDC, and Nascimento CP, et al (2022) The effectiveness of radiotherapy in preventing disease recurrence after breast cancer surgery Surg Oncol 41 101709 https://doi.org/10.1016/j.suronc.2022.101709 PMID: 35124329
45. Elston CW and Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up Histopathology 19(5) 403–410 https://doi.org/10.1111/j.1365-2559.1991.tb00229.x PMID: 1757079
46. Shousha S and Peston D (1997) Immunohistochemical demonstration of oestrogen and progesterone receptors in paraffin sections of breast carcinoma Curr Diagn Pathol 4(3) 176–180 https://doi.org/10.1016/S0968-6053(05)80006-7
47. Sannino P and Shousha S (1994) Demonstration of oestrogen receptors in paraffin wax sections of breast carcinoma using the monoclonal antibody 1D5 and microwave oven processing J Clin Pathol 47(1) 90–92 https://doi.org/10.1136/jcp.47.1.90 PMID: 8132819 PMCID: 501768
48. Mickey RM and Greenland S (1989) The impact of confounder selection criteria on effect estimation Am J Epidemiol 129(1) 125–137 https://doi.org/10.1093/oxfordjournals.aje.a115101 PMID: 2910056
49. Amin MB, Greene FL, and Edge SB, et al (2017) The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging CA Cancer J Clin 67(2) 93–99 https://doi.org/10.3322/caac.21388 PMID: 28094848
50. Giuliano AE, Edge SB, and Hortobagyi GN (2018) Eighth edition of the AJCC cancer staging manual: breast cancer Ann Surg Oncol 25(7) 1783–1785 https://doi.org/10.1245/s10434-018-6486-6 PMID: 29671136
51. Paik S, Shak S, and Tang G, et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer N Engl J Med 351(27) 2817–2826 https://doi.org/10.1056/NEJMoa041588 PMID: 15591335
52. Prat A, Cheang MC, and Martin M, et al (2013) Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer J Clin Oncol 31(2) 203–209 https://doi.org/10.1200/JCO.2012.43.4134 PMCID: 3532392
53. Ehinger A, Malmstrom P, and Bendahl PO, et al (2017) Histological grade provides significant prognostic information in addition to breast cancer subtypes defined according to St Gallen 2013 Acta Oncol 56(1) 68–74 https://doi.org/10.1080/0284186X.2016.1237778
54. Aleskandarany MA, Rakha EA, and Macmillan RD, et al (2011) MIB1/Ki-67 labelling index can classify grade 2 breast cancer into two clinically distinct subgroups Breast Cancer Res Treat 127(3) 591–599 https://doi.org/10.1007/s10549-010-1028-3
55. Liang Q, Ma D, and Gao RF, et al (2020) Effect of Ki-67 expression levels and histological grade on breast cancer early relapse in patients with different immunohistochemical-based subtypes Sci Rep 10(1) 7648 https://doi.org/10.1038/s41598-020-64523-1 PMID: 32376868 PMCID: 7203155
56. Fan Y, Ding X, and Xu B, et al (2015) Prognostic significance of single progesterone receptor positivity: a comparison study of estrogen receptor negative/progesterone receptor positive/Her2 negative primary breast cancer with triple negative breast cancer Medicine (Baltimore) 94(46) e2066 https://doi.org/10.1097/MD.0000000000002066
57. Zhao H and Gong Y (2021) The prognosis of single hormone receptor-positive breast cancer stratified by HER2 status Front Oncol 11 643956 https://doi.org/10.3389/fonc.2021.643956 PMID: 34079755 PMCID: 8165305
58. Bae SY, Kim S, and Lee JH, et al (2015) Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer BMC Cancer 15 138 https://doi.org/10.1186/s12885-015-1121-4 PMID: 25880075 PMCID: 4396721
59. Li Y, Yang D, and Yin X, et al (Clinicopathological characteristics and breast cancer-specific survival of patients with single hormone receptor-positive breast cancer) JAMA Netw Open 3(1) e1918160 PMID: 31899528 PMCID: 6991239
60. Li Z, Tu Y, and Wu Q, et al (2020) Clinical characteristics and outcomes of single versus double hormone receptor-positive breast cancer in 2 large databases Clin Breast Cancer 20(2) e151–e63 https://doi.org/10.1016/j.clbc.2019.07.002
61. Allison KH, Hammond ME, and Dowsett M, et al (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update J Clin Oncol 38(12):1346–1366 https://doi.org/10.1200/JCO.19.02309 PMID: 31928404
62. Burstein HJ (2020) Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer N Engl J Med 383(26) 2557–2570 https://doi.org/10.1056/NEJMra1307118 PMID: 33369357
63. Kunc M, Biernat W, and Senkus-Konefka E (2018) Estrogen receptor-negative progesterone receptor-positive breast cancer “Nobody’s land” or just an artifact? Cancer Treatment Rev 67 78–87 https://doi.org/10.1016/j.ctrv.2018.05.005
64. Mohammed H, Russell IA, and Stark R, et al (2015) Progesterone receptor modulates ERα action in breast cancer Nature 523(7560) 313–317 https://doi.org/10.1038/nature14583 PMID: 26153859 PMCID: 4650274
65. Metzger Filho O, Ignatiadis M, and Sotiriou C (2011) Genomic grade index: an important tool for assessing breast cancer tumor grade and prognosis Crit Rev Oncol Hematol 77(1) 20–29 https://doi.org/10.1016/j.critrevonc.2010.01.011
66. Sotiriou C, Wirapati P, and Loi S, et al (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis J Natl Cancer Inst 98(4) 262–272 https://doi.org/10.1093/jnci/djj052 PMID: 16478745
67. Ivshina AV, George J, and Senko O, et al (2006) Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer Cancer Res 66(21) 10292–10301 https://doi.org/10.1158/0008-5472.CAN-05-4414 PMID: 17079448
68. Dalton LW, Page DL, and Dupont WD (1994) Histologic grading of breast carcinoma. A reproducibility study Cancer 73(11) 2765–2770 https://doi.org/10.1002/1097-0142(19940601)73:11<2765::AID-CNCR2820731119>3.0.CO;2-K PMID: 8194018
69. Longacre TA, Ennis M, and Quenneville LA, et al (2006) Interobserver agreement and reproducibility in classification of invasive breast carcinoma: an NCI breast cancer family registry study Mod Pathol 19(2) 195–207 https://doi.org/10.1038/modpathol.3800496
70. Ellis IO, Coleman D, and Wells C, et al (2006) Impact of a national external quality assessment scheme for breast pathology in the UK J Clin Pathol 59(2) 138–145 https://doi.org/10.1136/jcp.2004.025551 PMID: 16443727 PMCID: 1860326
71. Loi S, Haibe-Kains B, and Desmedt C, et al (2007) Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade J Clin Oncol 25(10) 1239–1246 https://doi.org/10.1200/JCO.2006.07.1522 PMID: 17401012
72. Arima N, Nishimura R, and Osako T, et al (2019) Ki-67 index value and progesterone receptor status can predict prognosis and suitable treatment in node-negative breast cancer patients with estrogen receptor-positive and HER2-negative tumors Oncol Lett 17(1) 616–622 PMID: 30655808 PMCID: 6313203
73. Flanagan MB, Dabbs DJ, and Brufsky AM, et al (2008) Histopathologic variables predict Oncotype DX recurrence score Mod Pathol 21(10) 1255–1261 https://doi.org/10.1038/modpathol.2008.54 PMID: 18360352
74. Cuzick J, Dowsett M, and Pineda S, et al (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer J Clin Oncol 29(32) 4273–4278 https://doi.org/10.1200/JCO.2010.31.2835 PMID: 21990413
75. Sestak I, Buus R, and Cuzick J, et al (2018) Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial JAMA Oncol 4(4) 545–553 https://doi.org/10.1001/jamaoncol.2017.5524 PMID: 29450494 PMCID: 5885222
76. Sgroi DC, Sestak I, and Cuzick J, et al (2013) Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population Lancet Oncol 14(11) 1067–1076 https://doi.org/10.1016/S1470-2045(13)70387-5 PMID: 24035531 PMCID: 3918681
77. Inwald EC, Klinkhammer-Schalke M, and Hofstadter F, et al (2013) Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry Breast Cancer Res Treat 139(2) 539–552 https://doi.org/10.1007/s10549-013-2560-8 PMID: 23674192 PMCID: 3669503
78. Abdel-Fatah TMA, Webb R, and Li R, et al (2019) Evidence that neoadjuvant anthracycline based combination chemotherapy (NACT) in breast cancer (BC) induces phenotypical changes which guides the optimal adjuvant therapy J Clin Oncol 37(15_suppl) 590 https://doi.org/10.1200/JCO.2019.37.15_suppl.590
79. Abdel-Fatah TMA, Webb R, and Walker J, et al (2020) Abstract P5-06-06: clinically significant changes of receptors status (ER, PR and HER2) after receiving neoadjuvant chemotherapy (NACT) in breast cancer (BC) predicts the prognosis and guides the choice of the optimal adjuvant therapy (AT): retesting of the receptor status should be mandatory Cancer Res 80(4 Supplement) P5-06-06
80. Jones RL, Salter J, and A’Hern R, et al (2009) The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer Breast Cancer Res Treat 2009;116(1) 53–68 https://doi.org/10.1007/s10549-008-0081-7
81. Lee HC, Ko H, and Seol H, et al (2013) Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response J Breast Cancer 16(4) 395–403 https://doi.org/10.4048/jbc.2013.16.4.395
82. Li C, Fan H, and Xiang Q, et al (2019) Prognostic value of receptor status conversion following neoadjuvant chemotherapy in breast cancer patients: a systematic review and meta-analysis Breast Cancer Res Treat 178(3) 497–504 https://doi.org/10.1007/s10549-019-05421-7 PMID: 31471838
83. Lim SK, Lee MH, and Park IH, et al (2016) Impact of molecular subtype conversion of breast cancers after neoadjuvant chemotherapy on clinical outcome Cancer Res Treat 48(1) 133–141 https://doi.org/10.4143/crt.2014.262 PMCID: 4720061
84. Saúde MD (2021) PORTARIA CONJUNTA Nº 5, DE 18 DE ABRIL DE 2019 – Aprova as Diretrizes Diagnósticas e Terapêuticas do Carcinoma de Mama 2019 [https://www.gov.br/saude/pt-br/assuntos/protocolos-clinicos-e-diretrizes-terapeuticas-pcdt/arquivos/2019/ddt_carcinoma_cancerde_mama.pdf] Date accessed: 27/07/21
85. Dowsett M, Nielsen TO, and A’Hern R, et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group J Natl Cancer Inst 103(22) 1656–1664 https://doi.org/10.1093/jnci/djr393 PMID: 21960707 PMCID: 3216967
86. Criscitiello C, Disalvatore D, and De Laurentiis M, et al (2014) High Ki-67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast cancer Breast 23(1) 69–75 https://doi.org/10.1016/j.breast.2013.11.007
87. Bogina G, Zamboni G, and Sapino A, et al (2012) Comparison of anti-estrogen receptor antibodies SP1, 6F11, and 1D5 in breast cancer: lower 1D5 sensitivity but questionable clinical implications Am J Clin Pathol 138(5) 697–702 https://doi.org/10.1309/AJCPLX0QJROV2IJG PMID: 23086770
88. Calhoun BC, Mosteller B, and Warren D, et al (2018) Analytical and clinical performance of progesterone receptor antibodies in breast cancer Ann Diagn Pathol 35 21–26 https://doi.org/10.1016/j.anndiagpath.2018.02.007 PMID: 29758480
89. Rakha EA, Lee AH, and Roberts J, et al (2012) Low-estrogen receptor-positive breast cancer: the impact of tissue sampling, choice of antibody, and molecular subtyping J Clin Oncol 30(23) 2929–2930 author reply 31 https://doi.org/10.1200/JCO.2012.43.2831 PMID: 22753914
90. Troxell ML, Long T, and Hornick JL, et al (2017) Comparison of Estrogen and progesterone receptor antibody reagents using proficiency testing data Arch Pathol Lab Med 141(10) 1402–1412 https://doi.org/10.5858/arpa.2016-0497-OA PMID: 28714765
91. Allred DC, Clark GM, and Molina R, et al (1992) Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer Hum Pathol 23(9) 974–979 https://doi.org/10.1016/0046-8177(92)90257-4 PMID: 1355464
92. Harvey JM, Clark GM, and Osborne CK, et al (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer J Clin Oncol 17(5) 1474–1481 https://doi.org/10.1200/JCO.1999.17.5.1474 PMID: 10334533






