Palliative Prognostic Index accuracy of survival prediction in an inpatient palliative care service at a Brazilian tertiary hospital
Mauricio Fernandes1, Tiago Pugliese Branco1, Maria Clara Navarro Fernandez1, Carolina Paparelli1, Mariana Sarkis Braz1, Carolina Sassaki Kishimoto1, Helena Maria de Freitas Medeiros1, Karen Ebina1, Luciana Regina Bertini Cabral1, Simone Nagashima1, Silvia Amaral de Avó Cortizo1, Fabíola Borges1, Mariana Ribeiro Monteiro1, Ana Beatriz Kinupe Abrahao1, Raphael Brandão Moreira2, Alze Pereira dos Santos Tavares1 and Pedro Nazareth Aguiar Jr1,3
1Americas Centro de Oncologia Integrado, São Paulo 01321-001, Brazil
2Hospital Moriah, São Paulo 04083-002, Brazil
3Faculdade de Medicina do ABC, Santo André 09060-870, Brazil
Abstract
Purpose: The Palliative Prognostic Index (PPI) was developed to improve survival prediction for advanced cancer patients. However, there is limited data about the PPI application in a real-world scenario. This study aimed to assess the accuracy of PPI > 6 in predicting survival of cancer inpatients.
Methods: A prospective observational cohort in an inpatient palliative care service at a tertiary hospital in São Paulo-SP, Brazil, between May 2011 and December 2018.
Results: We included 1,376 critically ill cancer inpatients. Patients were divided into three PPI subgroups: PPI ≤ 4, PPI 4–6, and PPI ≥ 6. Their respective medium overall survival values were 44 days (95% confidence interval [CI] 35.52–52.47), 20 days (95% CI 15.40–24.59), and 8 days (95% CI 7.02–8.98), (p < 0.001). PPI ≥ 6 predicted survival of <3 weeks with a positive predictive value (PPV) of 72% and an negative predictive value (NPV) of 68% (sensitivity 67%, specificity 72%). PPI > 4 predicted survival of <6 weeks with a PPV of 88% and an NPV of 36% (sensitivity 74%, specificity 59%). When PPI was <4, the mortality rate over 3 weeks was 39% with a relative risk (RR) of 0.15 (95% CI 0.11–0.20; p < 0.001), and the 6-week mortality rate was 63% with a RR of 0.18 (95% CI 0.13–0.25; p < 0.001) compared to PPI ≥ 4.
Conclusions: PPI was a good discriminator of survival among critically ill cancer inpatients and could assist in hospital discharge decision. PPI may help healthcare policymakers and professionals in offering high-quality palliative care to patients.
Keywords: advanced cancer, palliative care, prognostic index, critical illness
Correspondence to: Pedro Nazareth Aguiar Jr.
Email: pnajpg@hotmail.com
Published: 11/05/2021
Received: 23/10/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Physicians often overestimate the survival time of seriously ill patients. These inaccurate prognoses lead to substantial hospice underuse and later hospice enrollment in end-of-life settings [1, 2]. Hence, physicians require reliable and practical prognostic tools to improve survival prediction and optimize end-of-life care for these patients.
In recent years, the significant development and validated use of prognostic tools have improved the clinical prediction of survival [3]. These prognostic models are based on assessing laboratory variables, performance status, and other clinical variables.
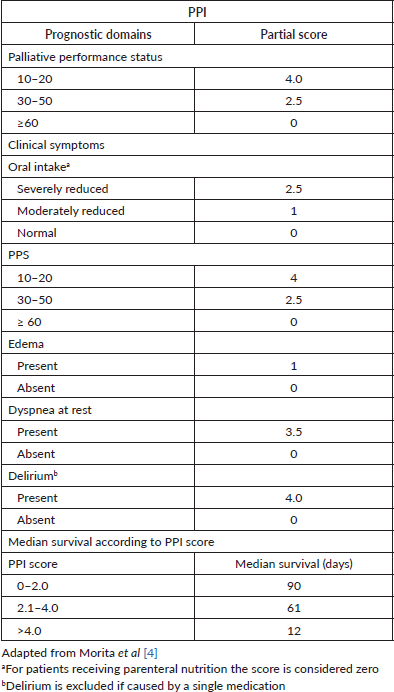
The Palliative Prognostic Index (PPI) is a well-known scoring system for predicting the survival of terminally ill cancer patients, and it was initially developed in a hospice inpatient unit in Japan [4]. PPI is calculated as the sum of the scores of the following variables: Palliative Performance Score (PPS), oral intake, edema, dyspnea at rest, and delirium (Table 1). This index was validated in several settings, from palliative care units to home care settings [5–9]. Significant advantages of PPI include relying only on clinical variables without the need for laboratory measures, simple utilization, and reproducible results [10].
Therefore, we aimed to evaluate PPI accuracy for predicting the survival of inpatients at a palliative care service in a Brazilian tertiary hospital. This study assessed a substantially extensive database of patients in a real-world scenario.
Methods
Study design and outcomes
We conducted this prospective observational study with a cohort of terminally ill cancer in patients who were referred for a palliative care service at a tertiary hospital and assessed the accuracy of PPI in predicting the survival and discussed its potential implications in clinical practice.
The data was collected prospectively under the study.
The primary endpoint was the accuracy of PPI > 6 in predicting survival of<3 weeks and PPI > 4 predicting survival of <6 weeks. These threshold values were similar to those present in the original publication by Morita et al [4]. Secondary endpoints were overall survival (OS) rates at 3 weeks with PPI > 6, the most accurate PPI scores to predict 6- and 3-week survival, 3-week mortality rate with PPI > 6, and 6-week mortality rate with PPI > 4.
This study followed the ethical principles and good practices of clinical research mentioned in Helsinki Declaration and Good Clinical Practice from the ICH, and local regulations, such as the Brazilian Resolution CNS/MS 466/12 and the Document of Americas 2005. The Paulistano Hospital institutional review board approved the study protocol. The Written Informed Consent Form application was waived because this was an observational study with information collected and registered in our database and also because the study did not affect patients’ usual care.
Patient eligibility and sampling method
The study had a convenience sampling approach with ill cancer inpatients referred for the Palliative Care Service at Paulistano Hospital, a private tertiary hospital in São Paulo-SP, Brazil, between May 2011 and December 2018.
The eligible participants could have any primary site tumor, including both hematologic and solid malignant neoplasms. Those with a potentially curable disease, i.e. candidates to definitive treatment, were considered not eligible. Eventually, we included 1,381 subjects in the study sample until December, 2018, the time to data cut-off. After excluding five patients whose PPI score was not available, 1,376 patients were included for the statistical analysis.
Data extraction
Data regarding patients’ characteristics, primary tumor location, performance status, clinical symptoms, and PPI scores were collected within 24 hours of the first referral to palliative care service. The palliative care physicians from our hospital received specific training to conduct this assessment.
Table 1. Description of components of PPI.
The patients were prospectively followed from the first assessment until death, decline in participation in the palliative care program, or transference to other hospitals. Patients’ survival was estimated from the first assessment to death or censored at the last contact with the care team.
Patients’ performance status was assessed with the PPS and [Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO)] scales. According to the original publication by Morita et al [4] PPI was measured as the sum of the scores of the PPS, oral intake, edema, dyspnea at rest, and delirium (Table 1). The PPI total score ranges from 0 to 15, and higher scores indicate a poorer general condition and worse survival.
The oral intake was subjectively assessed as normal, moderately reduced or severely reduced by the medical evaluator. In case the patient had received total or partial parenteral nutrition, he or she has been included in the normal oral intake group. Dyspnea at rest was evaluated due patient’s symptom referral or need of supplementary oxygen. Edema was documented as any swelling of limbs with indentation after gently thumb pressure during physical exam.
Delirium was diagnosed with the criteria from the American Psychiatric Association [11]. If the delirium was provoked only by a single medication and potentially reversible with the drug withdrawal, this finding was excluded from the final score sum.
Statistical analysis
Survival curves and 95% confidence intervals (CIs) of the median survival of the patients were calculated using the Kaplan–Meier method, and survival analyses were performed using the log-rank test. We arranged patients into three prognostic groups according to defined intervals: ≤4, 4–6, and ≥6 [4, 5].
We estimated the optimal PPI values for predicting 3- and 6-week survival based on receiver operating characteristics (ROC) curves and statistics. In this case, we included patients who either died or had a censored survival during the study.
The mortality rates at 3 and 6 weeks were evaluated according to different PPI cut-offs: PPI ≥ 6 versus PPI < 6 and PPI ≥ 4 versus PPI < 4. The statistical analysis of the odds ratio for mortality was performed using the chi-square test.
Among the patients who died, positive predictive values (PPVs) and negative predictive values (NPVs) were calculated for surviving <6 weeks with PPI > 4, and surviving <6 weeks with PPI > 6. Censored patients were not included in this analysis.
A significance level of 5% was considered for all statistical analyses. When information regarding PPI was not available, the patient was excluded from the statistical analysis for clinical outcomes. Statistical analyses were performed using IBM® SPSS Statistics version 24.
Results
Overall, 1,381 cancer patients were included in this study; their baseline characteristics are summarized in Table 2. Most patients had a reduced ECOG performance status of 3 or 4. The most frequent primary cancers were those of the lung/chest (17.2%), colorectal (14.3%), breast (11.2%), and pancreas/hepatobiliary system (13.4%).
At the time of analysis, an actual survival date was available for 1,037 patients (75.4%) who died, whereas censored survival was registered for the remaining 338 patients (24.6%). Because six patients underwent only an initial assessment, they were excluded from the survival analysis.
The study sample was divided into the three subgroups as follows: Group 1 included patients having PPI ≤ 4, Group 2 included those having PPI of 4–6, and Group 3 included those having PPI ≥ 6. The Kaplan–Meier survival curves for the three groups are shown in Figure 1. The median OS of Groups 1, 2, and 3 were 44 days (95% CI 35.52–52.47), 20 days (95% CI 15.40–24.59), and 8 days (95% CI 7.02–8.98), respectively, with a statistically significant difference after adjustment for primary site, age, and gender (p < 0.001).
3- and 6-week mortality rates
The primary endpoint was the accuracy of PPI > 6 in predicting survival of<3 weeks and PPI > 4 predicting survival of <6 weeks. When PPI was ≥6, the 3-week mortality rate was 86% with a RR (compared with PPI < 6) of 6.11 (95% CI 4.54–8.2; p < 0.001), and the 6-week mortality rate was 93% with a RR (compared with PPI < 6) of 5.44 (95% 3.72–7.96; p < 0.001) (Table 3).
Table 2. Patients baseline characteristics.
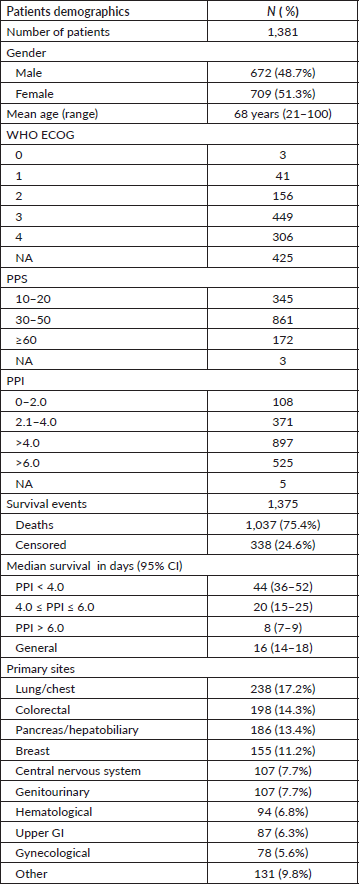
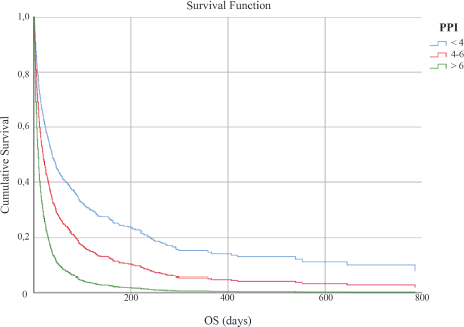
Figure 1. OS in days according to PPI score.
If PPI was ≥4, the 3-week mortality rate was 81% with a RR (compared with PPI < 4) of 6.68 (95% CI 4.44–7.38; p < 0.001), and the 6-week mortality rate was 91% with a RR (compared with PPI < 4) of 5.61 (95% CI 3.99–7.89; p < 0.01). Alternatively, when PPI was <4, the mortality rate over 3 weeks was 39% with a RR (compared with PPI ≥ 4) of 0.15 (95% CI 0.11–0.20; p < 0.001) and that over 6 weeks was 63% with a RR (compared with PPI ≥ 4) of 0.18 (95% CI 0.13–0.25; p < 0.001) (Table 3).
Compared to the PPI original publication [4], in our study a PPI ≥ 6 predicted a survival of <3 weeks with a PPV of 72% and an NPV of 68% (sensitivity 67%, specificity 72%). PPI > 4 predicted a survival of <6 weeks with a PPV of 88% and an NPV of 36% (sensitivity 74%, specificity 59%) (Table 4).
ROC curve
Using ROC curves of PPI, PPI <5.5 best predicted 6-week survival with a sensitivity of 79%, a specificity of 55%, and area under the curve (AUC) of 0.714 (Figure 2). PPI ≥5.5 best predicted 3-week mortality with a sensitivity of 67%, a specificity of 73%, and AUC of 0.753.
Discussion
Main findings
PPI is a useful scoring system for predicting the survival of terminally ill cancer patients in several settings, such as palliative care units and home care settings [5–9]. Significant advantages of this index include relying only on clinical variables without the need for laboratory measures, simple utilization, and reproducible results [10].
A PPI single measure has good accuracy for clinical practice with terminally ill patients; however, this might be improved with serial assessments [12]. If the patient’s clinical picture further deteriorates, it may be because of an increase in the PPI value [13].
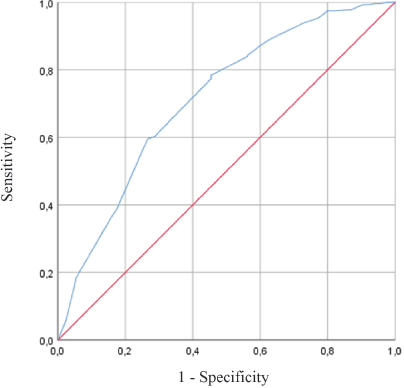
Figure 2. ROC Curve – PPI accuracy for survival prediction within 6 weeks.
Table 3. Analysis of 3-weeks mortality and 6-week mortality rates according to different PPI cut-offs in the study population including patients with confirmed death (excluding censored individuals).
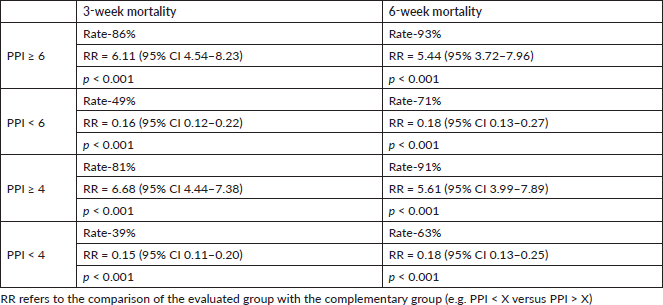
Table 4. Accuracy of predictions using the PPI – survival less than 3 weeks with PPI > 6 e survival less than 6 weeks with PPI > 4 – according to our study and Morita et al [4] original publication.

In our study, the prediction of survival of <6 weeks with PPI > 4 had high sensitivity and NPV, which were comparable to those reported in the original publication by Morita et al [4] However, specificity and PPV obtained in this study were slightly inferior to those reported in Morita et al [4] Alternatively, predicting survival of <3 weeks with PPI > 6 had inferior sensitivity and specificity values, NPVs, and PPVs compared with those reported in Morita et al [4]
Considering 3 and 6 weeks mortality rate, PPI equal or greater than 6 and equal or greater than 4 had almost identical results (86% and 93%. versus 81% and 91%, respectively). Conversely, the 3 weeks mortality among patients with PPI < 4 was only 39% and we consider that PPI < 4 can be considered a good prognostic factor.
Patients with PPI < 4 could likely benefit from a program and have an early hospital discharge. Moreover, clinicians should consider other factors, such as the primary site and treatment perspective, for decision-making regarding the care plan of patients with low PPI values. On the other hand, PPI ≥ 4 could be used as an indicator of a high likelihood of death and could help clinicians provide the best supportive care for patients.
We also performed a ROC curve analysis in order to find another PPI cut-off accurate and that include a larger number of patients in an early hospital discharge program validated in a future study. We found that a PPI cut-off of 5.5 would be the most accurate value for survival prediction within 3 and 6 weeks.
Strengths and limitations
Our study’s strengths were the large sample size, prospective design, and diversity of primary cancer sites. In addition, the study database included a great number of inpatients evaluated by the palliative service in our institution and over a long-term period. This extended study duration has led the care team to a better understanding and proper use of PPI.
In contrast, our study’s limitations were the single-center study design and restriction of the study population to include inpatients under evaluation by a palliative care service from a tertiary hospital. The reality is that convenience sampling highly likely led to a biased sample, which had a predominance of seriously ill subjects and refractory symptoms. Hence, these results would be less applicable to an outpatient or hospice scenario.
However, the study context reflects the current healthcare, which is hospital-centered, expensive, and ineffective, whereas palliative care and hospice remain new concepts [14].
Clinical relevance
This study prospectively assessed PPI’s effectiveness in a large cohort of cancer inpatients from a real-world scenario; patients enrolled in a palliative care team in a Brazilian tertiary hospital were included in this study. The routine use of PPI has been previously demonstrated to improve the clinical survival prediction of terminally ill cancer inpatients [15].
In this study, we demonstrated that PPI was a reliable and useful tool for predicting survival of critically ill cancer inpatients. Therefore, PPI will help physicians in making decisions regarding clinical practice and end-of-life care for cancer patients.
Conclusion
PPI is a useful scoring system for predicting the survival of terminally ill cancer patients in several settings. PPI’s significant advantages include relying only on clinical variables, without the need for laboratory measures, simple utilization, and reproducible results. This study results demonstrated that PPI is a good discriminator of survival among critically ill cancer inpatients, and it could assist in the decision about hospital discharge. PPI may help healthcare policymakers and professionals in providing high-quality optimized palliative care for patients.
Conflicts of interest and funding
All authors have no conflicts of interest to declare and the study was funded by the authors.
References
1. Gramling R, Gajary-Coots E, and Cimino J, et al (2019) Palliative care clinician overestimation of survival in advanced cancer: disparities and association with end-of-life care J Pain Symptom Manage 57 233–240 https://doi.org/10.1016/j.jpainsymman.2018.10.510
2. Christakis NA (2000) Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study BMJ 320 469–473 https://doi.org/10.1136/bmj.320.7233.469 PMID: 10678857 PMCID: 27288
3. Simmons CPL, McMillan DC, and McWilliams K, et al (2017) Prognostic tools in patients with advanced cancer: a systematic review J Pain Symptom Manage 53 962–970.e10 https://doi.org/10.1016/j.jpainsymman.2016.12.330 PMID: 28062344
4. Morita T, Tsunoda J, and Inoue S, et al (1999) The palliative prognostic index: a scoring system for survival prediction of terminally ill cancer patients Support Care Cancer 7 128–133 https://doi.org/10.1007/s005200050242 PMID: 10335930
5. Stone CA, Tiernan E, and Dooley BA (2008) Prospective validation of the palliative prognostic index in patients with cancer J Pain Symptom Manage 35 617–622 https://doi.org/10.1016/j.jpainsymman.2007.07.006 PMID: 18261876
6. Abk A, Hospital TP, and Rede P, et al (2015) Palliative prognostic index application at an inpatient palliative care service from a Brazilian tertiary hospital: a prospective observational study authors 30 2015
7. Sonoda H, Yamaguchi T, and Matsumoto M, et al (2014) Validation of the palliative prognostic index and palliative prognostic score in a palliative care consultation team setting for patients with advanced cancers in an acute care hospital in Japan Am J Hosp Palliat Med 31 730–734 https://doi.org/10.1177/1049909113506034
8. Hamano J, Kizawa Y, and Maeno T, et al (2014) Prospective clarification of the utility of the palliative prognostic index for patients with advanced cancer in the home care setting Am J Hosp Palliat Med 31 820–824 https://doi.org/10.1177/1049909113504982
9. Inomata M, Hayashi R, and Tokui K, et al (2014) Usefulness of the palliative prognostic index in patients with lung cancer Med Oncol 31 1–4 https://doi.org/10.1007/s12032-014-0154-x
10. Baba M, Maeda I, and Morita T, et al (2015) Survival prediction for advanced cancer patients in the real world: a comparison of the palliative prognostic score, delirium-palliative prognostic score, palliative prognostic index and modified prognosis in palliative care study predictor model Eur J Cancer 51 1618–1629 https://doi.org/10.1016/j.ejca.2015.04.025 PMID: 26074396
11. American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders 4th edn, (DSM-IV-TR)
12. Arai Y, Okajima Y, and Kotani K, et al (2014) Prognostication based on the change in the palliative prognostic index for patients with terminal cancer J Pain Symptom Manage 47 742–747 https://doi.org/10.1016/j.jpainsymman.2013.05.011
13. Kao CY, Hung YS, and Wang HM, et al (2014) Combination of initial palliative prognostic index and score change provides a better prognostic value for terminally ill cancer patients: a six-year observational cohort study J Pain Symptom Manage 48 804–814 https://doi.org/10.1016/j.jpainsymman.2013.12.246 PMID: 24709367
14. Hawley P (2017) Barriers to Access to Palliative Care [Internet] (Thousand Oaks: SAGE Publications Ltd) [http://pmc/articles/PMC5398324/] Date accessed: 22/03/21
15. Morita T, Tsunoda J, and Inoue S, et al (2011) Improved accuracy of physicians’ survival prediction for terminally ill cancer patients using the palliative prognostic index Palliat Med 15 419–424 https://doi.org/10.1191/026921601680419474






