Lymphopenia during chemoradiation—foe or friend
Vijay M Patil1#, Gunjesh Kumar Singh1#, Vanita Noronha1, Amit Joshi1, Nandini Menon1, Sarbani Ghosh Lashkar2, Vijayalakshmi Mathrudev1, Kavita Nawale Satam1, Sadaf Abdulazeez Mukadam1 and Kumar Prabhash1*
1Department of Medical Oncology, Tata Memorial Hospital, Mumbai 400012, India
2Department of Radiotherapy, Tata Memorial Hospital, Mumbai 400012, India
#Co-first author
Abstract
Background: Severe lymphopenia during treatment is considered to be a poor prognostic factor. The current literature lacks information regarding its impact on various outcomes in locally advanced head-and-neck cancer patients in a prospective setting.
Methods: We recently published a randomised study comparing cisplatin–radiation with nimotuzumab cisplatin–radiation. The database of this study was used for the present analysis. The impact of severe lymphopenia (grade 4 lymphopenia) on progression-free survival (PFS), locoregional control (LRC) and overall survival (OS) was studied using the Kaplan–Meier method and Cox regression analysis. The binary logistic regression analysis was used to see the effect of various factors on the development of severe lymphopenia.
Results: We had a total of 536 patients, of which 521 patients (97.7%) developed lymphopenia. Grade 1 lymphopenia was noted in 10 (1.9%) patients, grade 2 in 100 (18.8%), grade 3 in 338 (63.1%) and grade 4 in 73 (13.7%) patients. The median PFS was 20.53 and 60.33 months in severe and non-severe lymphopenia, respectively (hazard ratio, 0.797; p-value = 0.208). The median duration of LRC was 56.3 months in severe lymphopenia, whereas it was not reached in non-severe lymphopenia (hazard ratio, 0.81; p-value = 0.337). The median OS was 28.46 versus 47.13 months in severe and non-severe lymphopenia, respectively (hazard ratio, 0.76; p-value = 0.11). Of various risk factors, gender was significantly associated with severe lymphopenia.
Conclusion: The occurrence of severe lymphopenia was not significantly associated with the outcomes. Gender is the only risk factor significantly linked to severe lymphopenia.
Keywords: lymphopenia, head-and-neck cancer, chemoradiation
Correspondence to: Kumar Prabhash
Email: kumarprabhashtmh@gmail.com
Published: 24/09/2020
Received: 09/05/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Lymphocytes play a key role in tumour response; however, they are also the most sensitive cells to chemoradiation [1]. According to Campian et al [2], lymphopenia is commonly seen during the administration of chemoradiation to head-and-neck cancer (HNC) patients. In a prospective study, combined treatment with cisplatin and radiotherapy induced lymphopenia in 78% of patients [3]. In another phase I trial, lymphopenia (grade ≥ 3) was seen in 90% of patients receiving nimotuzumab along with chemoradiation for oesophageal cancer [4]. It has been postulated that lymphopenia hampers the body’s ability to destroy tumour cells [5].
HNC shows the presence of many infiltrating immune cells including lymphocytes—hence, it is called an immunogenic tumour [6]. Furthermore, the pre-treatment lymphocyte counts can be of prognostic value in HNC as proposed by some previous studies [7]. Reportedly, low absolute numbers of CD3 , CD4 and CD8 T cells have been found in patients with head-and-neck squamous cell carcinoma in comparison to the normal controls. In addition, patients with active disease tend to show significantly lower CD3 and CD4 T cell counts than those with no disease [2].
The occurrence of lymphopenia and its impact on outcomes have never been evaluated in any prospective study in HNC patients. In view of this lacuna in the current literature, we performed this post hoc analysis to study the impact of severe lymphopenia on outcome (progression-free survival, loco-regional control and overall survival) in HNC patients undergoing radical chemoradiation.
Materials and methods
Data set—We have recently published the results, outcome and adverse effect of a phase III randomised trial. In this study, 536 patients were randomised 1:1 to receive either radical radiotherapy (66–70 greys) with concurrent weekly cisplatin (30 mg/m2) (CRT) or the same schedule of CRT with weekly nimotuzumab (200 mg) (NCRT). The primary endpoint was progression-free survival (PFS); key secondary endpoints were the duration of loco-regional control (LRC) and overall survival (OS) [8]. The database of this study was used for the present post hoc analysis.
Data collection—From the above-mentioned data set, the data of lymphopenia in addition to demography and outcome were extracted, and the following were noted:
• The occurrence of lymphopenia from week 0 to week 6 of treatment was taken into account.
• The maximum grade of lymphopenia as per the CTCAE criteria 4.03.
• Absolute lymphocyte counts during chemoradiation.
Definition of lymphopenia as per the CTCAE criteria 4.03
• Grade 1 lymphopenia: < lower limit of normal—800/μL
• Grade 2 lymphopenia: 500–800/μL
• Grade 3 lymphopenia: 200–500/μL
• Grade 4 lymphopenia: <200/μL
Grade 4 lymphopenia was considered to be severe lymphopenia, whereas others (grade 1–3) were considered as non-severe lymphopenia.
Endpoints (outcomes)—To ascertain the impact of lymphopenia, the following endpoints were considered:
• PFS was defined as duration from the date of randomisation to the first evidence of tumour progression.
• LRC was defined as the time between the date of randomisation and the date of local or regional relapse.
• OS was calculated as the time from the date of randomisation to the date of death from any cause.
Statistics
Statistical analysis was performed using the SPSS version 20 and R version 3.5.3. A descriptive analysis was performed. The continuous variables were expressed in median value along with its range, whereas the non-continuous/ordinal variables were expressed in percentage (%) along with 95% confidence interval (CI). The impact of severe lymphopenia on PFS, LRC and OS was studied using the Kaplan–Meier method and Cox regression analysis. The binary logistic regression analysis was used to see the impact of factors (age, gender, Eastern Cooperative Oncology Group performance score (ECOG PS) and chemotherapy regimen) on the development of severe lymphopenia. A p-value of 0.05 was considered to be significant.
Results
Baseline characteristics
The detailed baseline characteristics of these patients have already been published [8]. The median age was 54 and 55 years in the CRT and NCRT arm, respectively. In the CRT arm, there were 86.2% of males and 13.8% of females, whereas, in NCRT arm, there were 84.3% of males and 15.7% of females. Stage IVA (64.2% versus 66%) was the most common stage, followed by stage III (32.5% versus 29.9%) and stage IVB (3.4% versus 4.1%) in CRT and NCRT, respectively. In both the arms, 99.6% of patients had ECOG PS of grade 0–1, whereas 0.4 % had grade 2. The baseline characteristics and treatment details of the patients are shown in Table 1.
Lymphopenia
The acute onset of lymphopenia was captured in 532 of these 536 patients. Of 532 patients, 521 patients (97.7%) developed lymphopenia. Grade 1 lymphopenia was noted in 10 (1.9%) patients, grade 2 in 100 (18.8%), grade 3 in 338 (63.5%) and grade 4 in 73 (13.7%) patients. Week-wise incidence and grade of lymphopenia are shown in Figure 1.
Factors impacting lymphopenia
Amongst various factors, only gender was found to be significantly associated with grade 4 lymphopenia (Table 2). Severe lymphopenia was seen in 12.6% of males (56 out of 454) and 22.4% of females (17 out of 76) (odds ratio: 2.043; 95% CI: 1.108–3.766; p-value = 0.022).
Impact on outcome
The median duration of follow‐up of this study was 39.13 months. At the time of censoring, 38 (n = 73) and 196 (n = 459) patients experienced disease progression with severe and non-severe lymphopenia, respectively, and their median PFS was 20.53 (95% CI: 6.87–34.18) and 60.33 months (95% CI: NA), respectively (hazard ratio: 0.797; 95% CI: 0.56–1.12; p-value = 0.208) (Figure 2). Loco-regional failure was observed in 30 patients with severe lymphopenia (n = 73), whereas it was seen in 160 (n = 459) in those with non-severe lymphopenia. The median duration of LRC was 56.3 months (95% CI: 0–115.78) in severe lymphopenia, whereas it was not reached in non-severe lymphopenia (hazard ratio: 0.81; 95% CI: 0.55–1.21; p-value = 0.337) (Figure 3). There were 42 (n = 73) versus 197 (n = 459) deaths, and the median OS was 28.46 (95% CI: 11.64–45.28) versus 47.13 (95% CI: NA) months in severe and non-severe lymphopenia, respectively (hazard ratio: 0.76; 95% CI: 0.54–1.06; p-value = 0.11) (Figure 4).
Apart from this, we also did an additional analysis to see any impact of lymphopenia on the outcomes. Here, we compared combined grade 0–2 lymphopenia with combined grade 3–4 lymphopenia. Of 532 patients, 121 patients (22.74%) developed lymphopenia of grade 0–2, whereas grade 3-4 lymphopenia was seen in 411 (77.25%) patients. At the time of censoring, 51 (n = 121) patients with grade 0–2 and 183 (n = 411) patients with grade 3–4 lymphopenia experienced disease progression. The median PFS of grade 0–2 and grade 3–4 lymphopenia was 46.2 (95% CI: 22.4–NA) and 56.3 months (95% CI: 22.9–NA), respectively (p-value = 0.63) (Supplementary Figure 1). Loco-regional failure was observed in 44 patients with grade 0–2 lymphopenia (n = 121), whereas it was seen in 146 (n = 411) patients with grade 3–4 lymphopenia. The median duration of LRC did not reach in both grade 0–2 and grade 3–4 lymphopenias (p-value = 0.93) (Supplementary Figure 2). There were 56 (n = 121) versus 183 (n = 411) deaths, and the median OS was 48.1 (95% CI: 21.7–NA) versus 43.5 (95% CI: 31.6–60.6) months in grade 0–2 and grade 3–4 lymphopenia, respectively (p-value = 0.68) (Supplementary Figure 3).
Table 1. Baseline characteristics and treatment details.
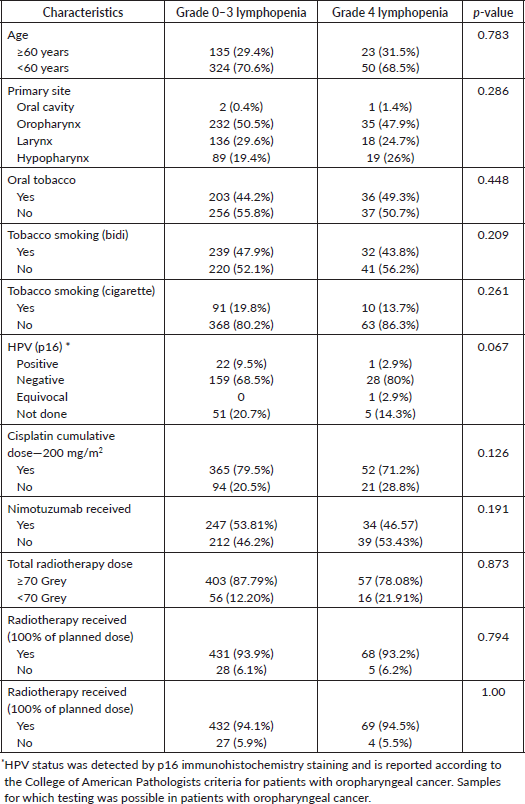
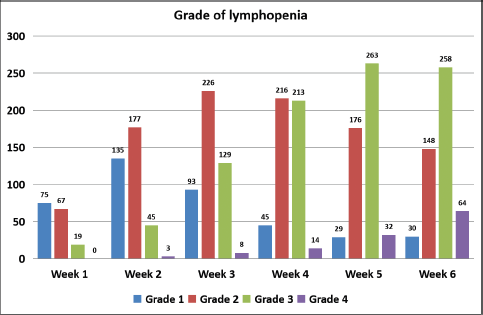
Figure 1. Week-wise incidence and grading of lymphopenia.
Table 2. Factors and their impact on severe lymphopenia
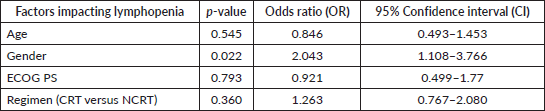
Figure 2. Kaplan–Meier curve showing progression-free survival in grade 4 (severe) and non-grade 4 lymphopenia (non-severe).
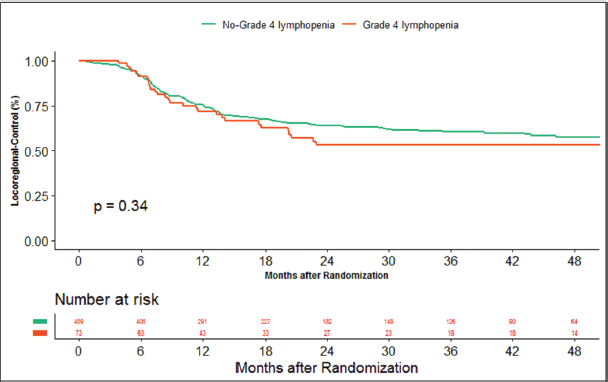
Figure 3. Kaplan–Meier curve showing loco-regional control in grade 4 (severe) and non-grade 4 lymphopenia (non-severe).
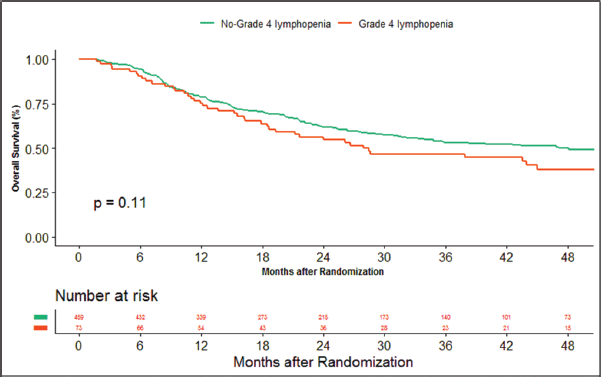
Figure 4. Kaplan–Meier curve showing overall survival in grade 4 (severe) and non-grade 4 lymphopenia (non-severe).
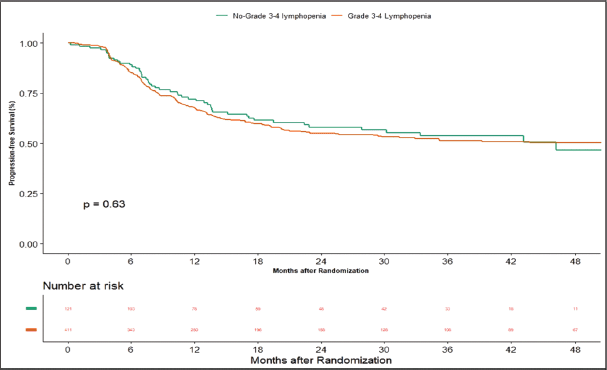
Supplementary Figure 1. Kaplan–Meier curve showing progression-free survival in grade 3–4 and non-grade 3–4 lymphopenia.
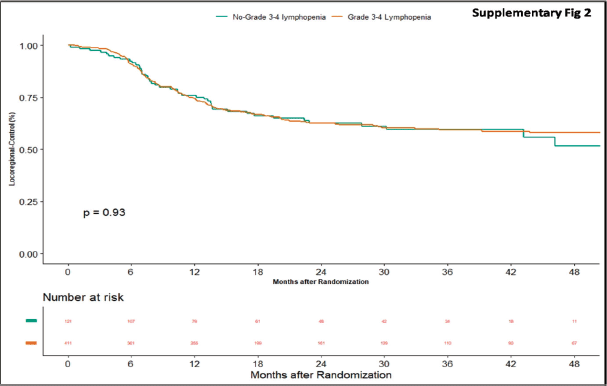
Supplementary Figure 2. Kaplan–Meier curve showing loco-regional control in grade 3–4 and non-grade 3–4 lymphopenia.
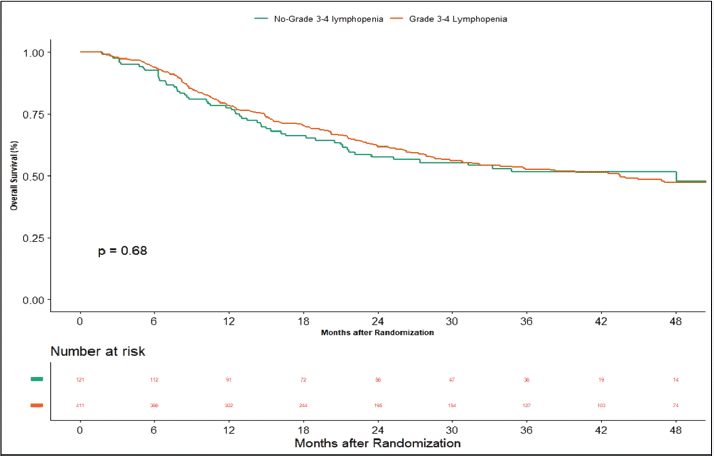
Supplementary Figure 3. Kaplan–Meier curve showing overall survival in grade 3–4 and non-grade 3–4 lymphopenia.
Discussion
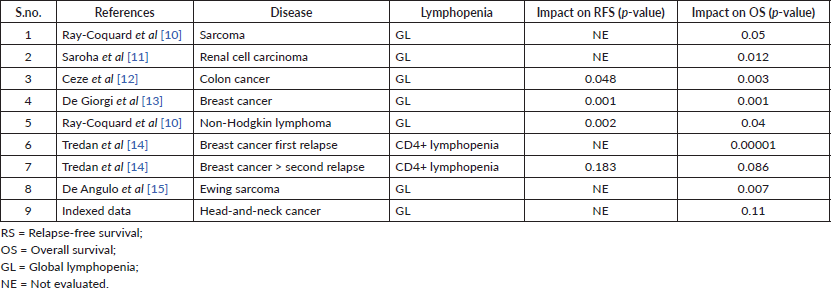
The existence of peripheral immune alterations in cancer patients was shown for the first time in the mid-1970s by Bone and Lauder [9]. Lymphopenia can be functional or treatment induced. Functional lymphopenia has been reported in advanced or metastatic breast cancer, colon cancer and hepatocellular carcinoma [7]. It is associated with dismal outcome. Furthermore, in various malignancies such as carcinoma breast, carcinoma ovary, non-Hodgkin lymphoma, diffuse large B-cell lymphoma, T-cell lymphomas, sarcomas and colon cancer, lymphopenia detected before treatment and early after starting chemotherapy (5–15 days) or radiotherapy is associated with a worse PFS and OS [7]. Its impact on relapse-free survival (RFS) and OS in various malignancies is shown in Table 3 [10–15]. In addition, the severity of lymphopenia has been reported as an independent prognostic factor of PFS and OS. According to Manuel et al [16], severe lymphopenia (<200/mm3) in metastatic breast carcinoma has a strong impact on patient survival and also reported 40% and 90% reduction of the median OS or PFS, respectively. Head-and-neck squamous cell carcinoma (HNSCC) is known as an immunogenic tumour showing the presence of infiltrating lymphocytes and other immune cells [17]. The previous studies have suggested the important role of pre-treatment lymphocyte counts with regard to their association with prognosis in HNSCC [7]. However, Campian et al [2] in a retrospective study found no significant difference in PFS and OS between patients with TLC < 500 cells/mm3 and those with TLC ≥ 500 cells/mm3. Similarly, in this study, there was no statistically significant impact of severe lymphopenia on various outcomes (PFS, LRC and OS).
Various studies have considered grade 3–4 as severe lymphopenia, and it was reported in 61% of HNC patients and 53% of cervical cancer patients by Campian et al [2] and Wu et al [18], respectively [18], whereas Zhou et al [17] found grade 4 lymphopenia in 31% of oesophageal cancer patients during chemoradiation. In this study, we found lymphopenia in 97.7% of patients, where, in 13.7% of patients, it was grade 4.
Older age, female gender, accelerated radiotherapy and certain chemotherapy agents (cisplatin, cyclophosphamide, methotrexate and taxanes) have been reported as significant risk factors for the development of lymphopenia [7, 19]. In this analysis, we analysed these factors. Of age, gender, ECOG performance score and chemotherapy regimen (cisplatin versus cisplatin nimotuzumab), gender was the only significantly associated factor with severe lymphopenia.
Table 3. Impact of lymphopenia on relapse-free survival (RFS) and overall survival (OS).
This first prospective study to enlighten lymphopenia and its impact on survival outcomes in head-and-neck cancer patients is unique. There are several limitations to this study. First of all, lymphopenia occurring only during treatment was analysed, so the post-therapy lymphopenia status cannot be commented on, although it is unlikely to witness post-treatment lymphopenia in a weekly chemotherapy schedule. Second, these data are applicable only for weekly cisplatin-based chemoradiation as there are different haematological toxicities in once a week versus once a 3-week cisplatin schedule [20].
Conclusion
Lymphopenia is fairly common in HNC patients during chemoradiation. Gender is an important factor associated with severe lymphopenia; however, there is no significant impact of severe lymphopenia on PFS, LRC and OS.
Conflicts of interest
None declared.
Declaration of funding
None.
References
1. Bremnes RM, Al-Shibli K, and Donnem T, et al (2011) The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer J Thorac Oncol 6 824–833 https://doi.org/10.1097/JTO.0b013e3182037b76
2. Campian JL, Sarai G, and Ye X, et al (2014) Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer Head Neck 36(12) 1747–1753 https://doi.org/10.1002/hed.23535 PMCID: 4081494
3. Visacri MB, Pincinato EC, and Ferrari GB, et al (2017) Adverse drug reactions and kinetics of cisplatin excretion in urine of patients undergoing cisplatin chemotherapy and radiotherapy for head and neck cancer: a prospective study Daru 25(1) 12 https://doi.org/10.1186/s40199-017-0178-9 PMID: 28438219 PMCID: 5404337
4. Kato K, Ura T, and Koizumi W, et al (2018) Nimotuzumab combined with concurrent chemoradiotherapy in Japanese patients with esophageal cancer: a phase I study Cancer Sci 109(3) 785–793 https://doi.org/10.1111/cas.13481 PMCID: 5834813
5. Grossman SA, Ellsworth S, and Campian J, et al (2015) Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors J Natl Compr Canc Netw 13(10) 1225–1231 https://doi.org/10.6004/jnccn.2015.0151 PMID: 26483062 PMCID: 4778429
6. Wolf GT, Hudson JL, and Peterson KA, et al (1986) Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis Otolaryngol Head Neck Surg 95 142–152 https://doi.org/10.1177/019459988609500203 PMID: 2954014
7. Wolf GT, Schmaltz S, and Hudson J, et al (1987) Alterations in T‐lymphocyte subpopulations in patients with head and neck cancer. Correlations with prognosis Arch Otolaryngol Head Neck Surg 113 1200–1206 https://doi.org/10.1001/archotol.1987.01860110066010 PMID: 3499156
8. Patil VM, Noronha V, and Joshi A, et al (2019) A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer Cancer 125(18) 3184–3197 https://doi.org/10.1002/cncr.32179 PMID: 31150120
9. Bone G and Lauder I (1974) Cellular immunity, peripheral blood lymphocyte count and pathological staging of tumours in the gastrointestinal tract Br J Cancer 30 215–221 https://doi.org/10.1038/bjc.1974.184 PMID: 4451626 PMCID: 2009205
10. Ray-Coquard I, Dussart S, and Goillot C, et al (2009) A risk model for severe anemia to select cancer patients for primary prophylaxis with epoetin alpha: a prospective randomized controlled trial of the ELYPSE study group Ann Oncol 20 1105–1112 https://doi.org/10.1093/annonc/mdn750 PMID: 19174452
11. Saroha S, Uzzo RG, and Plimack ER, et al (2013) Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma J Urol 189 454–461 https://doi.org/10.1016/j.juro.2012.09.166 PMCID: 3545104
12. Ceze N, Thibault G, and Goujon G, et al (2011) Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy Cancer Chemother Pharmacol 68 1305–1313 https://doi.org/10.1007/s00280-011-1610-3 PMID: 21448592
13. De Giorgi U, Mego M, and Scarpi E, et al (2012) Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer Clin Breast Cancer 12 264–269 https://doi.org/10.1016/j.clbc.2012.04.004 PMID: 22591634
14. Tredan O, Manuel M, and Clapisson G, et al (2013) Patients with metastatic breast cancer leading to CD4 T cell lymphopaenia have poor outcome Eur J Cancer 49 1673–1682 https://doi.org/10.1016/j.ejca.2012.11.028
15. De Angulo G, Hernandez M, and Morales-Arias J, et al (2007) Early lymphocyte recovery as a prognostic indicator for high-risk Ewing sarcoma J Pediatr Hematol Oncol 29 48–52 https://doi.org/10.1097/MPH.0b013e31802d3e3e PMID: 17230066
16. Manuel M, Tredan O, and Bachelot T, et al (2012) Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients Oncoimmunology 1 432–440 https://doi.org/10.4161/onci.19545 PMID: 22754761 PMCID: 3382902
17. Zhou XL, Zhu WG, and Zhu ZJ, et al (2019) Lymphopenia in esophageal squamous cell carcinoma: relationship to malnutrition, various disease parameters, and response to concurrent chemoradiotherapy Oncologist 24(8) e677–e686 https://doi.org/10.1634/theoncologist.2018-0723 PMID: 31040254 PMCID: 6693723
18. Wu ES, Oduyebo T, and Cobb LP, et al (2016) Lymphopenia and its association with survival in patients with locally advanced cervical cancer Gynecol Oncol 140 76–82 https://doi.org/10.1016/j.ygyno.2015.11.013 PMCID: 4782779
19. Terrones-Campos C, Ledergerber B, and Vogelius IR, et al Lymphocyte count kinetics, factors associated with the end-of-radiation-therapy lymphocyte count, and risk of infection in patients with solid malignant tumors treated with curative-intent radiation therapy Int J Radiat Oncol Biol Phys pii: S0360-3016(19)33495-9
20. Noronha V, Joshi A, and Patil VM, et al (2018) Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial J Clin Oncol 36(11) 1064–1072 https://doi.org/10.1200/JCO.2017.74.9457




