Local recurrence of renal cell carcinoma successfully treated with fusion imaging-guided percutaneous thermal ablation
Nicola Camisassia, Giovanni Mauri, Paolo Della Vigna, Guido Bonomo, Gianluca Maria Varano, Daniele Maiettini and Franco Orsi
Division of Interventional Radiology, IEO, European Institute of Oncology IRCCS, Milan 20141, Italy
ahttps://orcid.org/0000-0002-7697-5651
Abstract
Image-guided thermal ablations are increasingly applied in the treatment of renal cancers, under the guidance of ultrasound (US) or computed tomography (CT). Fusion imaging allows exploitation of the strengths of all imaging modalities simultaneously, eliminating or minimising the weaknesses of every single modality. We present a case of a 68-year-old patient treated using US/CT fusion imaging to guide radiofrequency ablation for local recurrence of renal cell carcinoma undetectable by ultrasound.
Keywords: imaging-guided ablation, renal cell carcinoma recurrence, fusion imaging, radiofrequency ablation
Correspondence to: Nicola Camisassi
Email: nicola.camisassi@ieo.it
Published: 13/07/2020
Received: 16/03/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Renal cell carcinoma (RCC) is the most common primary malignancy of the kidney, accounting for about 2% of all cancer diagnoses in humans [1]. Image-guided tumour ablation has been used increasingly in the treatment of patients with renal tumours, particularly in the case of lesions smaller than 4 cm, and in patients with single kidney with the aim of avoiding loss of renal function deriving from surgical nephrectomy [2]. The availability of the most advanced imaging techniques, including a dedicated room with computed tomography (CT) and a last generation ultrasound (US) machine, possibly equipped with fusion imaging, may allow the correct targeting of tumour not well visible by ultrasound alone and thus to maximise the technical result [3, 4]. We here present a case of a 68-year-old patient with previous nephrectomy successfully treated using US/CT fusion imaging to guide radiofrequency (RF) ablation for local recurrence of RCC undetectable by ultrasound.
Case report
A 68-year-old-male patient with a history of right nephrectomy was treated 3 months before by microwave ablation for RCC of the left kidney (Figure 1). He presented with local recurrence and was referred to our institution for treatment. Multi-detector computed tomography (MD-CT) showed the presence of a 15 mm hypervascular nodule, centrally located, on the inner margin of the previous ablation area (Figure 2). Despite the recurrence after the first treatment, surgery was still considered too risky since his solitary kidney so, after multidisciplinary discussion and agreement, another attempt of imaging-guided ablation was proposed as first line treatment. It was decided to use RF ablation combined with pyeloperfusion, in order to protect the collecting system from thermal damage. The procedure was performed under general anaesthesia in our Angio-CT Hybrid Suite with the availability of angiography, CT and US (Figure 3). First a 6 French stent (Pollack Open-End Flexi-Tip® Ureteral Catheter, Cook Urological) was placed into the ipsilateral renal pelvis (Figure 4). Then, we preliminarily performed an US examination to localise the lesion, but unfortunately, it was completely invisible on B-mode US due to its central location in the kidney, completely hidden behind the scar of the previous treatment. With patient in right lateral decubitus, MD-CT with contrast media was performed and data were transferred in DICOM format to the US system. Fusion imaging was used to guide the needle to target the residual tumour combining real-time US scans with CT images (Figure 5). Radiofrequency ablation (RFA) was performed with 3 cm umbrella-shaped multi-tines needle electrode (LeVeen CoAccess RFA needle electrode, Boston Scientific, MA, USA), selected to match the size of the tumour. The electrode was inserted and then connected to the generator (RF 3000, Boston Scientific) after checking the correct positioning with a CT scan; energy was applied till roll-off occurred. Roll-off was achieved three times (80 W at 11 min, 60 W at 8 minutes and 60 W at 10 minutes) before needle removal. During ablation, cold pyeloperfusion was performed with gentle injection of sterile water through the ureteral stent. Patient was discharged the day after in good conditions after a clinical evaluation and MD-CT performed to exclude complications. Follow-up CT scan performed at 6 weeks showed complete tumour ablation without enhancing residual tumour (Figure 6). Renal function did not significantly change at 6 months from the treatment (preoperative creatinine levels = 1.65 mg/dl, 6 months creatinine levels = 1.63 mg/dl)
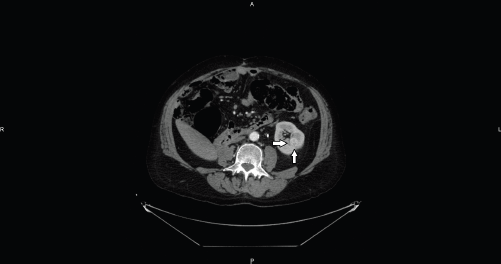
Figure 1. Axial CT image shows an intraparenchymal mid-renal hypervascular nodule referred to RCC (arrows).
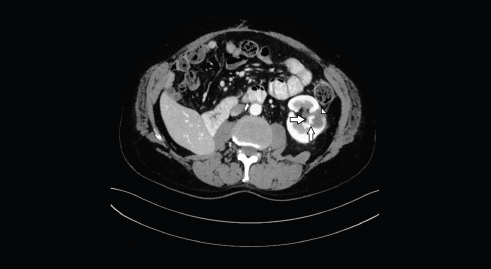
Figure 2. Axial CT image shows the hypervascular nodule (arrows), centrally located, on the inner margin of the previous ablation area (head of arrow).
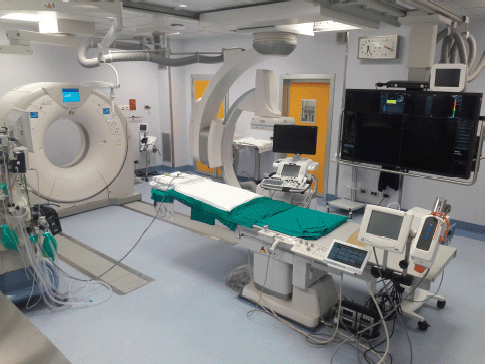
Figure 3. Angio-CT Hybrid Suite with the availability of angiography, CT and US.
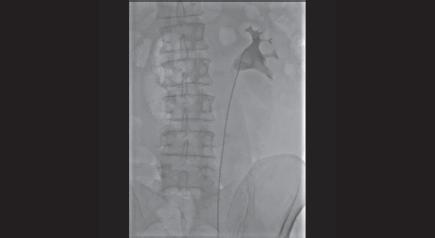
Figure 4. Retrograde pyelography shows the right positioning of the left ureteral stent in the omolateral collecting system.
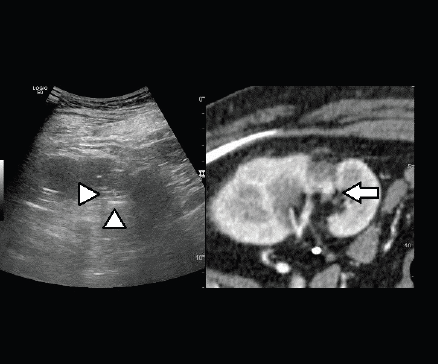
Figure 5. Fusion imaging combining real time US with CT images: CT scan shows the hypervascular nodule deeply in the scar of the previous treatment (arrow); the lesion is not clearly visible at US (heads of arrow).
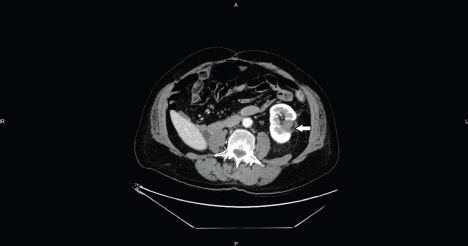
Figure 6. Axial CT image shows the hypo-enhancing ablation zone without enhancing residual tumor (arrow).
Discussion
RCC accounts for 2%–3% of all adult malignancies [5]. The detection of RCC is increasing owing to an increase in the diagnosis of small asymptomatic renal masses with cross-sectional imaging [5]. Radical nephrectomy represents the conventional treatment, while, more recently, surgical nephron sparing techniques have been developed with the aim of reducing invasiveness [6–8]. The application of image-guided ablative techniques to renal cancer has pushed in order to offer treatment to patient not suitable for surgery and to spare the highest amount of renal parenchyma [6, 9, 10] with reported good clinical results [11, 12]. The most widely used ablative techniques used to treat renal tumours are cryoablation, RF ablation and microwave ablation [13–15]. With RF an alternating current determines ionic friction that leads to slow heat generation, with subsequent protein denaturation, blood coagulation and coagulative necrosis [16, 17]. US is the most commonly used image guidance method worldwide to guide percutaneous thermal ablation due to its low cost, availability, high contrast resolution, absence of ionising radiation and real-time guidance capabilities in any imaging plane [18, 19]. However, it can be sometime difficult to distinguish between simple and complex or neoplastic cyst and to detect and characterise solid lesions so that a second level method such as CT or MRI is often required [20]. In case of lesions undetectable with ultrasound, imaging-guided ablation is often considered to be not feasible. In those cases, real-time fusion imaging of ultrasound and pre-acquired CT or MRI images has been reported as a feasible and an effective way to successfully detect the target lesion and perform percutaneous ablations [18, 21]. In a study of Auer et al [22] fusion imaging, combining real-time US and pre-acquired CT or MRI images, were performed successfully in the majority (89.3%) of patients in which either a kidney lesion of interest could not definitively be localised or sufficiently defined with grey-scale US alone. Contrast-enhanced ultrasound is also emerging as a useful technique to study cystic lesions and their related septal vascularisation, as well as solid lesions, during ablative procedures [23, 24]. However, Helck et al [25] have already described a more accurate identification of kidney lesions with fusion imaging compared to US and CEUS alone. When targeting renal lesions during imaging-guided ablation, fusion imaging can help in recognising the most appropriate part of the lesion to biopsy (especially, for cystic lesions) and distinguishing more precisely its margins in order to find the best position to place the electrodes for ablation, as in our case. Furthermore, the use of fusion imaging is valuable in determining the correct path for the needle, avoiding harm to structures such as the renal vessels, renal pelvis, adrenal glands, spleen and colon. In the case presented, it would have been not possible to treat the patient with thermal ablation without the application of fusion imaging, and the patient would have been treated by surgical nephrectomy, with consequent loss of renal function and need of dialysis for the rest of his life. With the application of fusion imaging in our dedicated hybrid angio suite, it has been possible to successfully treat the patient with RF ablation, with limited impact on renal function.
Conclusion
Especially when treating residual or recurrence of renal lesions, either after surgery or percutaneous thermal ablation, tumour could not be sufficiently defined with grey-scale US alone. The combination of real-time US scans with CT images is an essential tool to place applicators for ablation, thus offering to the patients a chance to be treated in a minimally invasive way.
Conflicts of interest statement
The authors declare that they have no conflicts of interest.
Funding declaration
The authors received no specific funding for this work.
References
1. Zagoria RJ (2000) Imaging of small renal masses: a medical success story AJR Am J Roentgenol 175 R945–R955 https://doi.org/10.2214/ajr.175.4.1750945
2. Breen DJ and Railton NJ (2010) Minimally invasive treatment of small renal tumors: trends in renal cancer diagnosis and management Cardiovasc Intervent Radiol 33(5) 896–908 https://doi.org/10.1007/s00270-010-9892-0 PMID: 20544228
3. Li W, Bai Y, and Wu M, et al (2016) Combined CT-guided radiofrequency ablation with systemic chemotherapy improves the survival for nasopharyngeal carcinoma with oligometastasis in liver: propensity score matching analysis Oncotarget 8(32) 52132–52141 https://doi.org/10.18632/oncotarget.10383 PMID: 28881719 PMCID: 5581018
4. Andersson M, Hashimi F, and Lyrdal D, et al (2015) Improved outcome with combined US/CT guidance as compared to US guidance in percutaneous radiofrequency ablation of small renal masses Acta Radiol 56 1519–1526 https://doi.org/10.1177/0284185114558974
5. Rini BI, Campbell SC, and Escudier B (2009) Renal cell carcinoma Lancet 373(9669) 1119–1132 https://doi.org/10.1016/S0140-6736(09)60229-4 PMID: 19269025
6. Mauri G, Nicosia L, and Varano GM, et al (2017) Tips and tricks for a safe and effective image-guided percutaneous renal tumour ablation Insights Imaging 8(3) 357–363 https://doi.org/10.1007/s13244-017-0555-4 PMID: 28500486 PMCID: 5438321
7. Herr H (2005) A history of partial nephrectomy for renal tumors J Urol 173 705–708 https://doi.org/10.1097/01.ju.0000146270.65101.1d PMID: 15711247
8. Ljungberg B, Cowan NC, and Hanbury DC, et al (2010) EAU guidelines on renal cell carcinoma: the 2010 update Eur Urol 58 398–406 https://doi.org/10.1016/j.eururo.2010.06.032 PMID: 20633979
9. Potretzke AM, Larson JA, and Bhayani SB (2015) Re: R. Houston Thompson, tom Atwell, grant Schmit, et al. comparison of Partial nephrectomy and percutaneous ablation for cT1 renal masses Eur Urol 2015;67:252–9 Eur Urol 67 e19–e20 https://doi.org/10.1016/j.eururo.2014.09.011
10. Gervais DA, McGovern FJ, Wood BJ et al (2000) Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology 217:665–672 https://doi.org/10.1148/radiology.217.3.r00dc39665 PMID: 11110926
11. Ahmed M, Brace CL, and Lee FT, et al (2011) Principles of and advances in percutaneous ablation Radiology 258 351–369 https://doi.org/10.1148/radiol.10081634 PMID: 21273519 PMCID: 6939957
12. Frey GT, Sella DM, and Atwell TD (2015) Image-guided renal intervention Radiol Clin N Am 53 1005–1019 https://doi.org/10.1016/j.rcl.2015.05.002 PMID: 26321450
13. Mauri G, Cova L, and Ierace T, et al (2016) Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation Cardiovasc Intervent Radiol 39 1023–1030 https://doi.org/10.1007/s00270-016-1313-6 PMID: 26911732
14. Cazzato RL, Garnon J, and Ramamurthy N, et al (2016) Percutaneous image-guided cryoablation: current applications and results in the oncologic field Med Oncol 33 1–16 https://doi.org/10.1007/s12032-016-0848-3
15. Shin BJ, Chick JF, and Stavropoulos SW (2016) Contemporary status of percutaneous ablation for the small renalmass Curr Urol Rep 17 23 https://doi.org/10.1007/s11934-016-0581-7
16. Kelly EF and Leveillee RJ (2016) Image guided radiofrequency ablation for small renal masses Int J Surg 36 525–532 https://doi.org/10.1016/j.ijsu.2016.11.026 PMID: 27847290
17. Balageas P, Cornelis F, and Le Bras Y, et al (2013) Ten-year experience of percutaneous image-guided radiofrequency ablation of malignant renal tumours in high-risk patients Eur Radiol 23 1925–1932 https://doi.org/10.1007/s00330-013-2784-3 PMID: 23443351
18. Mauri G, Cova L, and De Beni S, et al (2015) Real-Time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases Cardiovasc Intervent Radiol 38(1) 143–151 https://doi.org/10.1007/s00270-014-0897-y
19. Auer T, Heidegger I, and De Zordo T, et al (2019) Fusion imaging of contrast-enhanced ultrasound with CT or MRI for kidney lesions In vivo 33 203–208 https://doi.org/10.21873/invivo.11460 PMCID: 6364056
20. European Society of Radiology (ESR) (2019) Abdominal applications of ultrasound fusion imaging technique: liver, kidney, and pancreas Insight Imaging 10(1) 6 https://doi.org/10.1186/s13244-019-0692-z
21. Monfardini L, Gennaro N, and Della Vigna P, et al (2019) Cone-beam CT-assisted ablation of renal tumors: preliminary results Cardiovasc Intervent Radiol 42(12) 1718–1725 https://doi.org/10.1007/s00270-019-02296-5 PMID: 31367773
22. Hakime A, Yevich S, and Tselikas L, et al. (2017) Percutaneous thermal ablation with ultrasound guidance. Fusion imaging guidance to improve conspicuity of liver metastasis Cardiovasc Intervent Radiol 40(5) 721–727 https://doi.org/10.1007/s00270-016-1561-5 PMID: 28054165
23. Meloni MF, Smolock A, and Cantisani V, et al (2015) Contrast enhanced ultrasound in the evaluation and percutaneous treatment of hepatic and renal tumors Eur J Radiol 84(9) 1666–1674 https://doi.org/10.1016/j.ejrad.2015.06.004 PMID: 26094868
24. Gulati M, King KG, and Gill IS, et al (2015) Contrast enhanced ultrasound (CEUS) of cystic and solid renal lesions: a review Abdom Imaging 40(6) 1982–1996 https://doi.org/10.1007/s00261-015-0348-5 PMID: 25588715
25. Helck A, D’Anastasi M, and Notohamiprodjo M, et al (2012) Multimodality imaging using ultrasound image fusion in renal lesions Clin Hemorheol Microcirc 50(1–2) 79–89 https://doi.org/10.3233/CH-2011-1445 PMID: 22538537






