Relationship of CK8/18 expression pattern to breast cancer immunohistochemical subtyping in Egyptian patients
Hayam A Aiad1, Rehab M Samaka1, Nancy Y Asaad1, Mona A Kandil1, Mohamed A Shehata2 and Islam M Miligy1
1 Department of Pathology, Faculty of Medicine, Menoufia University, 32511, Egypt
2 Department of Oncology, Faculty of Medicine, Menoufia University, 32511, Egypt
Correspondence to: Hayam A Aiad. Email: hayam.aiad@yahoo.com
Abstract
The immunohistochemical (IHC) subtyping of breast cancer can be a useful substitute for gene expression analysis. The aim of this study was to investigate the relationship of CK8/18 to the biology of breast carcinoma (BC) represented by its IHC subtypes.
The IHC expression of CK8/18 was correlated with IHC subtypes of BC using ER, PR, HER2/neu, and Ki67 LI (with cutoff 14%). All cases showed CK 8/18 expression in tumour cells with varying degree of intensities; 49/70 cases (70%) showed diffuse cytoplasmic expression (loss of membranous pattern), while 21/70 cases (30%) showed membrano-cytoplasmic pattern. Adjacent non-neoplastic breast lobules showed membrano-cytoplasmic pattern in 58% of cases, which was significantly different from the pattern in invasive cancer (P = 0.002). A loss of membranous pattern in malignant tumours was significantly associated with higher tumour grade (P = 0.02), higher mitotic count (P = 0.03), and negative HER2/neu status (P = 0.04). CK 8/18 H score ranged between 1 and 290 with mean ± SD was 181 ± 70.54. Tumours with lower CK 8/18 H score were in the advanced stage group (P = 0.04). Low CK8/18 H score and loss of membranous pattern were significantly associated with triple negative (TN) subtype as compared with luminal subtype (P = 0.006 and P = 0.026, respectively). In addition, CK8/18 with lost membranous pattern was significantly associated with TN subtype compared with HER2/neu positive subtype (P = 0.001). However, there was no significant difference between luminal A and B subtypes regarding CK8/18 H score or pattern of expression. This study concluded that low CK8/18 H score and loss of membranous pattern of CK8/18 are associated with worse prognostic features and TN subtype.
Keywords: CK8/18, Ki67 labelling index, immunohistochemical subtyping, breast carcinoma
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast carcinoma (BC) in Egypt shows poor prognostic features, such as higher stage and higher grade than in developed countries, which may be attributed to different lifestyle and genetic factors [1].
Attention has been directed at molecular classification of breast cancer to stratify patients into well-defined prognostic categories that can be used in management decision [2, 3]. Standard immunohistochemical (IHC) tests (ER, PR, HER2, and Ki67) when used in a combinatorial manner can provide similar information as expensive molecular assays [4–6].
Keratins are epithelial-specific intermediate filament proteins, which are expressed in a tissue-specific manner [7]. CK18, a type I intermediate filament protein, and its co-expressed complementary type II partner, CK8, are persistently expressed in a variety of adult epithelial organs including breast and are also expressed by cancers that arise from these tissues [8]. However, regulatory changes in cytokeratins expression challenged the view that cytokeratins are only marker proteins [9]. Hypothetical mechanism for the role of CK18 in carcinogenesis states that CK18 acts as an identical target of Akt in the phosphoinositide 3-kinase pathway protecting cells from apoptosis [10, 11] and of extracellular signal-regulated kinase (ERK1/2) in the ERK mitogen-activated protein kinase pathway [12], and regulation of CK18 by Wnt is involved in Akt activation [13, 14].
This study was designed to evaluate the IHC expression of CK8/18 in BC of Egyptian patients to identify their relevance for IHC subtyping.
Material and methods
This retrospective study was conducted on archival tissue from 70 BCs of Egyptian patients. The cases were diagnosed in the Pathology Department, Faculty of Medicine, Menoufia University, spanning the period between January 2007 and December 2010. Selection was based on the availability of paraffin-embedded blocks for serial cutting and examination.
Histopathological assessment
Grading was performed according to the modified Bloom–Richardson method [15]. Scoring of mitosis was carried out using an Olympus CH2 light microscope with wide angle (field size: 0.274 mm2, field diameter: 0.59 mm). The mitotic figures were carefully defined to avoid inclusion of apoptotic cells. The mitotic activity index was calculated as the total number of figures counted in ten HPF fields of vision. The same cutoff as in previous publications was chosen, with ≥ 10 mitoses defined as high risk [16]. Nottingham prognostic index (NPI) is calculated according to Galea et al [17]. Grade I and II were lumped into one group for statistical purposes.
Staging was done based on the tumour node metastasis system [18]. For statistical purpose, cases were lumped into two groups: early stage—including stages I and II and advanced stage—including stages III and IV.
IHC staining
IHC staining was performed using LAB-SA (labelled {strept} Avidin–Biotin) immunoenzymatic antigen detection system (Lab Vision/Neo Markers, California, United States). Antigen retrieval was done by boiling in citrate buffer saline (pH, 6), followed by cooling at room temperature. The primary antibodies were incubated overnight at room temperature. For CK 8/18, mouse monoclonal antibody keratin 8/18 Ab-2 ready-to-use was used (clone K8.8 DC10; like 5D3, Lab Vision, Neo Marker). For Ki67, primary rabbit polyclonal anti-Ki67 antibody, MIB1 clone, M7240 was used and diluted 1:300 (DakoCytomation, Copenhagen, Denmark). Positive control slides were prepared by staining skin carcinoma (for CK 8/18) and tonsil (for Ki67). For oestrogen receptor (ER), primary antibody against ER was used (clone 1D5; Dilution, 1:50) (DakoCytomation). For progesterone receptor (PR), primary antibody against PR was used (clone IA6; Dilution, 1:50) (DakoCytomation). For HER2/neu: primary antibody against HER2/neu was used (clone 250, Dilution, 1:100) (DakoCytomation). BC cases previously known to be positive for ER, PR, and HER2/neu were used as positive control slides. Negative control slides were also included in each run and were done by the replacement of primary antibody by antibody diluents. Secondary antibody was applied with diaminobenzidine as a chromogen substrate and Mayer’s haematoxylin as a counter stain.
Immunostaining interpretation
IHC staining of CK8/18 was evaluated in non-neoplastic and invasive cancer breast tissue concerning the pattern of expression either cytoplasmic, membranous or both [19]. H score was also calculated for CK8/18 using the intensity and percentage of positive cells [20]. The intensity score (0–3) was multiplied by the percentage of cells that stain with each level of intensity and the sum of these mathematical products was expressed as a score of 0–300. H score formula = strong intensity (3) × percentage moderate intensity (2) × percentage mild intensity (1) × percentage [20].
The Ki67 LI was determined using a semi-quantitative visual approach. Scoring was performed while blinded to patients’ information and outcomes. The entire slide was scanned for immunostaining evaluation using light microscope at low-power magnification (×100). All tumour cell nuclei with homogenous granular staining, multiple speckled staining or nucleolar staining were regarded as positively stained, regardless of intensity, while any cytoplasmic immunoreactivity was considered non-specific and hence not taken into consideration. Scoring was performed in the areas with highest number of positive nuclei (hot spot) within the invasive component of the tumour. The Ki67 LI (tumour growth fraction) was expressed as the percentage of Ki67-positive malignant cells among a total number of 1000 malignant cells, at high-power magnification (×400) [21]. Using 14% as an optimal cutoff point, cases were classified into low and high proliferative groups [22]. Histological grade II was also stratified into low and high proliferative subgroups: GIIa and GIIb [21].
ER and PR were considered positive if ≥ 1% of tumour cell nuclei are immunoreactive [23]. HER2/neu immunoreactivity was evaluated according to the American Society of Clinical Oncology guideline recommendations [24]. Positive HER2/neu cases were defined as 3 positivity (>30% intense and complete staining); however, score 0 or 1 was considered negative and 2 cases were excluded.
According to the IHC results, ER, PR, HER2/neu, and Ki67 LI, we classified our cases into groups equivalent to molecular subtypes; luminal A subtype: ER and/or PR , HER 2/neu−, with low Ki67 LI, luminal B1subtype: ER and/or PR , HER 2/neu− with high Ki67 LI, luminal B2 subtype: ER and/or PR , HER 2/neu with any Ki67 LI, HER2/neu positive subtype: ER−, PR−, and HER2/neu positive, and finally triple negative (TN) subtype: ER− PR− HER 2 neu− [22].
Statistical analysis
Data were statistically analysed using a personal computer with the Software Package for Statistical Analysis version 16 program; (USA). Chi-square test (X2 test) and Fisher’s exacts test were used to compare qualitative variables. Student t-test and Mann–Whitney (U test) were used to compare quantitative variables. Tests were considered statistically significant when (P ≤ 0.05) and highly significant when (P < 0.01).
Results
The mean age of malignant cases was 49.97 ± 11.08, ranged between 25 and 81 years. The mean tumour size was 4.04 ± 1.95 ranged between 0.5 and 11 cm; 61/70 cases (87%) were invasive duct carcinoma of no special type (invasive carcinoma and no special type). The remaining cases were special types 9/70 (13%) including, four invasive lobular carcinoma, three medullary carcinoma, and two mixed tubular carcinomas.
Three out of 70 cases showed histological grade I (4%), 39/70 cases (56%) were grade II, and 28/70 cases were grade III (40%); 57/70 cases (81%) showed lymph node metastasis, 51/70(73%) cases showed advanced stage, 40/70 cases (57%) showed poor NPI, and 39/70 cases (56%) showed necrosis. The mean mitotic count was 5.56 ± 4.76 for all the cases ranged between 0 and 22. ER positive cases were 46/70 (66%), PR positive cases were 40/70 (57%), and HER2/neu positive cases were 22/70 (31%).
CK8/18 immunostaining
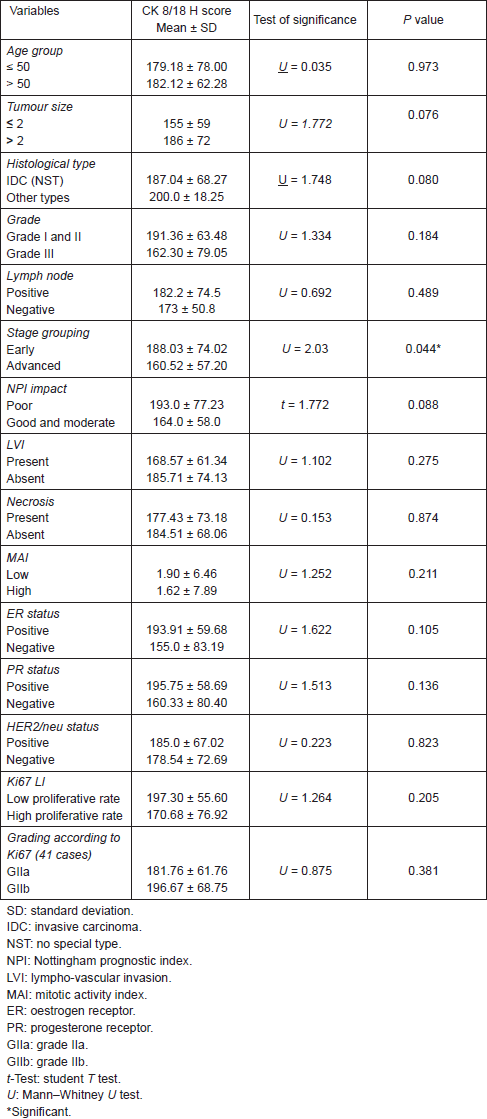
All cases showed CK 8/18 expression in tumour cells with varying degree of intensities; 49/70 cases (70%) showed diffuse cytoplasmic expression (loss of membranous pattern; Figure 1), while 21/70 cases (30%) showed membrano-cytoplasmic pattern (Figure 2). Non-neoplastic breast tissue adjacent to invasive cancer was observed in 52 cases. Membrano-cytoplasmic pattern was observed in 30/52 (58%; Figure 3), which was significantly different from pattern in invasive cancer (P = 0.002). Loss of membranous pattern in malignant tumours was significantly associated with higher tumour grade (P = 0.02), higher mitotic count (P = 0.03), and negative HER2/neu status (P = 0.04; Table 1). CK 8/18 H score ranged between 1 and 290 with mean ± SD was 181 ± 70.54. Tumours with lower CK 8/18 H score belonged to the advanced stage group (P = 0.04; Table 2).
According to Ki67 LI, histological grade II was further stratified into low and high proliferative subgroups: GIIa comprised 17 cases (41.5%) and GIIb 24 cases (58.5). There was no significant difference between both groups regarding CK 8/18 pattern of expression or H score (Tables 1 and 2).
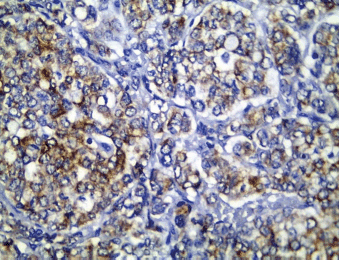
Figure 1. CK 8/18 cytoplasmic staining (loss of membranous pattern) in invasive duct carcinoma (immunoperoxidase ×400).
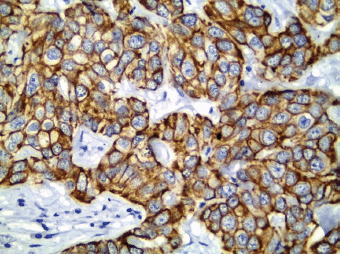
Figure 2. CK 8/18 with preserved membranous pattern in invasive duct carcinoma (immunoperoxidase ×400).
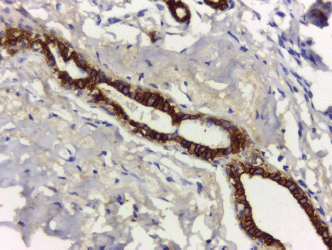
Figure 3. Non-neoplastic breast tissue shows membrano-cytoplasmic pattern (immunoperoxidase ×200).
Table 1. Association between CK 8/18 pattern of expression and the clinico-pathological parameters.
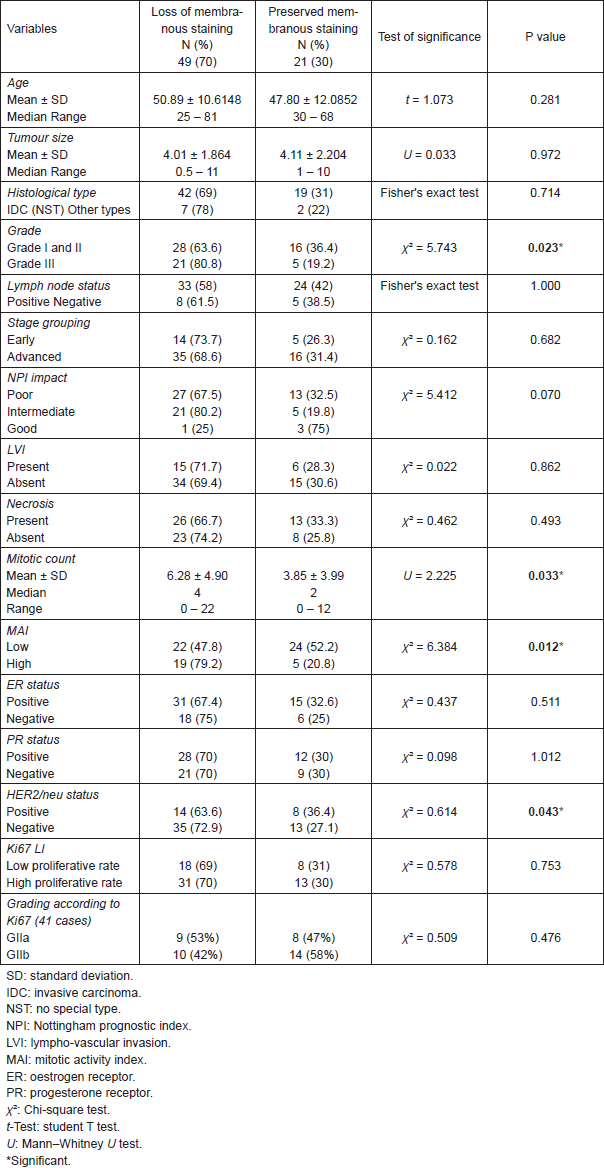
Table 2. Association between CK 8/18 H score and different clinico-pathological features.
IHC subtyping of carcinoma cases
Luminal A subtype represented 35 cases (50%), luminal B2 subtype: 11 cases (15.71%), HER2/neu positive subtype: 11 cases (15.71%), and TN subtype: 13 cases (18.58%). However, there was no case included in luminal B1 subtype. The luminal group was significantly associated with low histological grade, absent necrosis, and low mitotic count when compared with HER2/neu and TN groups.
Comparison between different subtypes of BC cases, clinico-pathological parameters and CK8/18 IHC expression (Table 3)
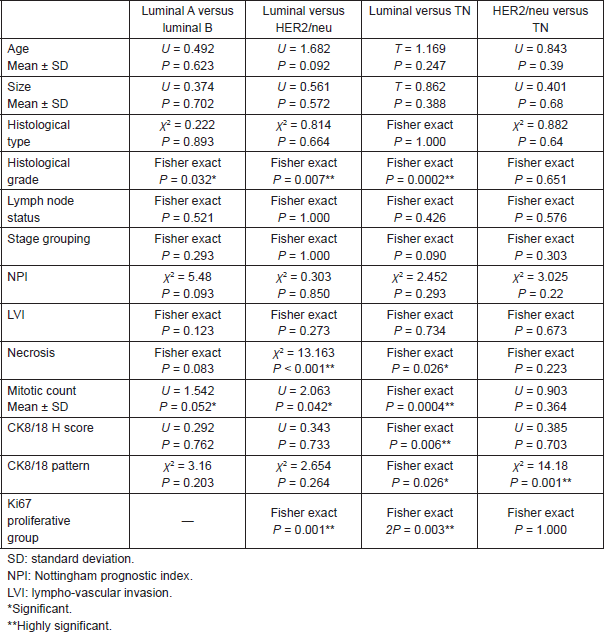
High CK8/18 H score and preserved membranous pattern were significantly associated with luminal group as compared to TN group (P = 0.006 and P = 0.026, respectively). Luminal A subtype was significantly associated with more differentiated tumours (P = 0.03) and lower mitotic count (P = 0.05) when compared with luminal B subtype. However, CK8/18 H score or pattern of expression did not show a significant difference between both luminal groups.
There was no significant difference between HER2/neu and TN groups regarding the clinico-pathological features, Ki67 LI or CK8/18 H score. However, preserved membranous pattern of CK8/18 was significantly associated with HER2/neu positive group when compared with TN group (P = 0.001).
Table 3. Multiple comparisons between immunohistochemical subtypes.
Discussion
IHC subtyping of BC provides valuable information for clinical decision making. However, it is highly dependent on training, skills, and experience of laboratory personnel performing it. In addition variability in outcomes still present among patients of a particular subtype according to the current calcification system [25]. Therefore, there is an increasing need for additional refinement of prognostic factors to improve patient risk stratification. Cytokeratins have long been considered as markers for identifying epithelial origin [9]. In this study, we prove that CK8/18 has a strong relationship to biology of BC represented in its IHC subtypes.
In this study, CK 8/18 was expressed in all BC cases with varying degrees of intensity and percentage. Lower CK 8/18 H score showed significant association with advanced stage (P = 0.04). Similarly, low CK 8/18 expression in breast cancer cell lines was associated with high metastatic potential [26], and loss of these keratins was associated with a significantly worse prognosis [27]. The more interesting finding is the pattern of CK8/18 expression; we identified two main patterns of staining: membrano-cytoplasmic (30% of cases) and cytoplasmic patterns (70% of cases). We suggest a significant relationship between CK 8/18 pattern of expression and tumour behaviour, which may be explained by the subcellular localisation and the biological functions of CK 8/18. We claim that preserved membranous localisation (membrano-cytoplasmic pattern), which was significantly more observed in non-neoplastic breast lobules, may reflect a degree of differentiation that maintain the tissue integrity and resist stresses externally applied to the cell [28].
In contrast, we observed that loss of membranous pattern of CK 8/18 (cytoplasmic only) was significantly associated with higher tumour grade (P = 0.05), higher mean mitotic count (P = 0.033), and high proliferative group (P = 0.012). CK 8/18 is important for cellular processes, such as cell signalling [29], mitosis [30], cell cycle progression [31], and protection from apoptosis [10, 11]. Thus, it is involved in intracellular signalling pathways that lead to cell cycle progression which may explain the worse prognostic features associated with predominant cytoplasmic localisation with loss of membranous pattern of CK 8/18. Similarly, Cîmpean et al [19] observed three different distribution patterns of CK8/18 expression: diffuse cytoplasmic, membranous, and combined granular cytoplasmic with membranous but they did not correlate them to different clinico-pathological features.
One of the features common to BC is the increased rate of proliferation over that observed in normal breast epithelia [32]. It is now acknowledged that increased cell proliferation is a key determinant of poor outcome in patients with breast cancer [21, 33, 34].
Different cutoff points for Ki67 LI were tried to identify the valid prognostic one (10%, 14%, and 20%) [21, 22, 35]. In this study, we have assessed proliferative activity using Ki67 LI with the cutoff point of 14% because it is the best cutoff point to distinguish luminal B from luminal A tumours [22, 36].
Based on the IHC results of ER, PR, HER2/neu, and Ki67 LI, we classified our cases into four groups equivalent to molecular subtypes (luminal A, luminal B, HER2/neu positive, and TN subtypes). The IHC subtypes in the present series were comparable with the Western and Egyptian studies; 50% belonged to luminal A subtype in comparison with other studies: 41.2% [37], 53.1% [38], and 59.1% [39]. Luminal B subtype constituted 15.7% of cases compared with other studies: 13.9 % [37], 16.4 % [39], and 21.7% [38]. HER2/neu positive subtype constituted 15.71% compared with 8.5% [40], 12.7% [39], and 9% [38]. Finally, 18.58% were of TN subtype in comparison with 14.5% [40], 11.8% [39], and 16.2% [38].
As expected, the luminal subtype showed better clinico-pathological features when compared with HER2/neu and TN subtypes. We aimed to find out whether evaluation of CK8/18 is correlated to IHC classification of BC. CK8/18 has been called luminal marker as it indicates normal luminal epithelial-like differentiation [41]. CK 8/18 was expressed in all the studied BC cases including luminal and non-luminal subtypes. Our results indicate that the mere positivity of CK8/18 does not discriminate between luminal and non-luminal subtypes of BC. Therefore, we found that decreased CK8/18 H score and loss of membranous pattern was associated with TN subtype when compared with luminal and HER2/neu subtypes. These findings emphasise on the role of CK8/18 in the tumour biology of BC.
It has been suggested that luminal B subtype is equivalent to those that express either HER2/neu or Ki67 [35]. In this study luminal B was categorised as those showing Ki67 LI > 14% and all were found to be positive for HER2/neu coinciding with those called B2 subtype [22]. According to our results, the existence of B1 group, a subset of luminal B subtype, that shows negativity for HER2/neu in Egyptian population is questioned.
In this study, there was a statistical significant association between luminal B subtype and both higher tumour grade and higher mitotic count when compared with luminal A subtype. This coincides with poor prognosis associated with luminal B as compared with luminal A tumours [42–44]. However, there was no significant difference between luminal A and B subtypes with respect to CK8/18 expression.
There was no significant difference between the clinico-pathological features of HER2/neu and TN subtypes. In addition, there was no significant difference between both groups regarding Ki67 LI group. This agrees with previous reports that indicated that proliferation markers are of limited value in the TN and HER2/neu positive tumours as the majority of these tumours are poorly differentiated with a high proliferation index [45]. As regards to CK8/18 pattern of expression, loss of membranous pattern was significantly associated with TN group. This may further help in the differentiation between both groups since the treatment strategies differ.
The major limitation of this study is the small number of cases with available paraffin blocks suitable for recutting and immunostaining. This is because our hospital is a local centre with limited resources of archiving, documentation, and follow-up of cases. Moreover, in Egypt, we do not have a national wide program neither for breast cancer public awareness nor for screening. Therefore, cases are lately diagnosed and referred to the more equipped National Cancer Institute in Cairo. In spite of this limitation, our results indicate that the mere positivity of CK8/18 does not discriminate between luminal and non-luminal subtypes of BC; however, low CK8/18 H score and loss of membranous pattern of staining are associated with worse prognostic features and TN subtype.
Conflicts of interest
No funding was received from any organisation.
Ethical approval was not required.
References
1. Rennert G (2006) Breast cancer ed L Freedman et al Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared with US SEER (Bethesda: National Cancer Institute (NIH)). Pub. No. 06-5873
2. Perou CM et al (2000) Molecular portraits of human breast tumours Nature 406(6797) 747–52 DOI: 10.1038/35021093 PMID: 10963602
3. Sørlie T et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications Proc Natl Acad Sci USA 98(19) 10869–74 DOI: 10.1073/pnas.191367098 PMID: 11553815 PMCID: 58566
4. Cuzick J et al (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer J Clin Oncol 29(32) 4273–8 DOI: 10.1200/jco.2010.31.2835 PMID: 21990413
5. Voduc KD et al (2010) Breast cancer subtypes and the risk of local and regional relapse J Clin Oncol 28(10) 1684–91 DOI: 10.1200/jco.2009.24.9284 PMID: 20194857
6. Skarlos P et al (2012) Triple-negative phenotype is of adverse prognostic value in patients treated with dose-dense sequential adjuvant chemotherapy: a translational research analysis in the context of a Hellenic Cooperative Oncology Group (HeCOG) randomized phase III trial Cancer Chemother Pharmacol 69(2) 533–46 DOI: 10.1007/s00280-011-1730-9
7. Moll R et al (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells Cell 31(1) 11–24 DOI: 10.1016/0092-8674(82)90400-7 PMID: 6186379
8. Oshima RG, Baribault H and Caulín C (1996) Oncogenic regulation and function of keratins 8 and 18 Cancer Metastasis Rev 15(4) 445–71 DOI: 10.1007/bf00054012 PMID: 9034603
9. Choi I, Gudas LJ and Katzenellenbogen BS (2000) Regulation of keratin 19 gene expression by estrogen in human breast cancer cells and identification of the estrogen responsive gene region Mol Cell Endocrinol 164(1–2) 225–37 DOI: 10.1016/s03037207(00)00197-0 PMID: 11026574
10. Ku NO et al (2010) Cytoskeletal keratin glycosylation protects epithelial tissue from injury Nat Cell Biol 12(9) 876–85 DOI: 10.1038/ncb2091 PMID: 20729838 PMCID: 3549664
11. Rotty JD, Hart GW and Coulombe PA (2010) Stressing the role of O-GlcNAc: linking cell survival to keratin modification Nat Cell Biol 12(9) 847–9 DOI: 10.1038/ncb0910-847 PMID: 20811358
12. Zhang XS et al (2006) Dedifferentiation of adult monkey Sertoli cells through activation of extracellularly regulated kinase 1/2 induced by heat treatment Endocrinology 147(3) 1237–45 DOI: 10.1210/en.2005-0981
13. Ohigashi T et al (2005) Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells Prostate 62(1) 61–8 DOI: 10.1002/pros.20117
14. Yee DS et al (2010) The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition Mol Cancer 9 162 DOI: 10.1186/1476-4598-9-162 PMID: 20573255 PMCID: 2907330
15. Elston CW and Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up Histopathology 19(5) 403–10 DOI: 10.1111/j.1365-2559.1991.tb00229.x PMID: 1757079
16. Baak JP et al (2005) Prospective multicenter validation of the independent prognostic value of the mitotic activity index in lymph node-negative breast cancer patients younger than 55 years J Clin Oncol 23 5993–6001 DOI: 10.1200/jco.2005.05.511 PMID: 16135467
17. Galea MH et al (1992) The Nottingham prognostic index in primary breast cancer Breast Cancer Res Treat 22 207–19 DOI: 10.1007/bf01840834 PMID: 1391987
18. Edge SB, Byrd DR and Compton CC (ed) (2010) Breast AJCC Cancer Staging Manual 7th edn (New York, NY: Springer) pp 347–76
19. Cîmpean AM et al (2008) Relevance of the immunohistochemical expression of cytokeratin 8/18 for the diagnosis and classification of breast cancer Rom J Morphol Embryol 49(4) 479–83
20. Bilalovic N et al (2004) CD10 protein expression in tumor and stromal cells of malignant melanoma is associated with tumor progression Mod Pathol 17(10) 1251–8 DOI: 10.1038/modpathol.3800174 PMID: 15205682
21. Aleskandarany MA et al (2011) MIB1/Ki-67 labelling index can classify grade 2 breast cancer into two clinically distinct subgroups Breast Cancer Res Treat 127(3) 591–9 DOI: 10.1007/s10549-010-1028-3
22. Goldhirsch A et al (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011 Ann Oncol 22(8) 1736–47 DOI: 10.1093/annonc/mdr304 PMID: 21709140 PMCID: 3144634
23. Hammond ME et al (2010) American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer Arch Pathol Lab Med 134 907–22 PMID: 20524868 PMCID: 3073033
24. Wolff AC et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer J Clin Oncol 25 118–45 DOI: 10.1200/jco.2006.09.2775
25. Alizart M et al (2012) Molecular classification of breast carcinoma Diagn Histopathol 18(3) 97–103 DOI: 10.1016/j.mpdhp.2011.12.003
26. Schaller G et al (1996) Elevated keratin 18 protein expression indicates a favorable prognosis in patients with breast cancer Clin Cancer Res 2(11) 1879–85 PMID: 9816144
27. Becker M et al (2002) Sensitive PCR method for the detection and real-time quantification of human cells in xenotransplantation systems Br J Cancer 87(11) 1328–35 DOI: 10.1038/sj.bjc.6600573 PMID: 12439725 PMCID: 2408903
28. Coulombe PA and Wong P (2004) Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds Nat Cell Biol 6(8) 699–706 DOI: 10.1038/ncb0804-699 PMID: 15303099
29. Coulombe PA and Omary MB (2002) ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments Curr Opin Cell Biol 14(1) 110–22 DOI: 10.1016/s0955-0674(01)00301-5 PMID: 11792552
30. Ku NO et al (2002) Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression Proc Natl Acad Sci USA 99 4373–8 DOI: 10.1073/pnas.072624299 PMID: 11917136 PMCID: 123655
31. Galarneau L et al (2007) Keratins modulate hepatic cell adhesion, size and G1/S transition Exp Cell Res 313(1) 179–94 DOI: 10.1016/j.yexcr.2006.10.007
32. Whitfield ML et al (2006) Common markers of proliferation Nat Rev Cancer 6(2) 99–106 DOI: 10.1038/nrc1802 PMID: 16491069
33. van Diest PJ, van der Wall E and Baak JP (2004) Prognostic value of proliferation in invasive breast cancer: a review J Clin Pathol 57(7) 675–81 DOI: 10.1136/jcp.2003.010777 PMID: 15220356 PMCID: 1770351
34. de Azambuja E et al (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients Br J Cancer 96(10) 1504–13 DOI: 10.1038/sj.bjc.6603756 PMID: 17453008 PMCID: 2359936
35. Wiesner FG et al (2009) Ki-67 as a prognostic molecular marker in routine clinical use in breast cancer patients Breast 18(2) 135–41 DOI: 10.1016/j.breast.2009.02.009 PMID: 19342238
36. Cheang MC et al (2009) J Natl Cancer Inst 101(10) 736–50 DOI: 10.1093/jnci/djp082 PMID: 19436038 PMCID: 2684553
37. El-Hawary AK et al (2012) Molecular subtypes of breast carcinoma in Egyptian women: clinicopathological features Pathol Res Pract 208(7) 382–6 DOI: 10.1016/j.prp.2012.03.011 PMID: 22641056
38. Park S et al (2011) Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry Breast 21 50–7 DOI: 10.1016/j.breast.2011.07.008 PMID: 21865043
39. Adly S, Hewedi IH and Mokhtar NM (2010) Clinicopathologic significance of molecular classification of breast cancer: relation to nottingham prognosis index J Egypt Natl Canc Inst 22(4) 209–15
40. Hugh J et al (2009) Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical de finition in the BCIRG 001 trial J Clin Oncol 27 1168–76 DOI: 10.1200/jco.2008.18.1024 PMID: 19204205 PMCID: 2667821
41. Abd El-Rehim DM et al (2004) Expression of luminal and basal cytokeratins in human breast carcinoma J Pathol 203(2) 661–71 DOI: 10.1002/path.1559 PMID: 15141381
42. Blows FM et al (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies PLoS Med 7(5) 1–12 DOI: 10.1371/journal.pmed.1000279
43. Anderson H et al (2011) Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer Ann Oncol 22 1770–76 DOI: 10.1093/annonc/mdq700 PMID: 21285137
44. Chuah BYS et al (2011) Serial changes in the expression of breast cancer-related proteins in response to neoadjuvant chemotherapy Ann Oncol 22 1748–54 DOI: 10.1093/annonc/mdq755 PMID: 21355070
45. Wirapati P et al (2008) Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures Breast Cancer Res 10(4) R65 DOI: 10.1186/bcr2124 PMID: 18662380 PMCID: 2575538






