Comparison analysis of serum interleukin-6 levels and cervical cancer
Muisi A Adenekan1, Joseph B Minari2, Ayodeji Adefemi3, Gbenga Olorunfemi4,5, Ayomide I Fayinto6, Adebayo Sekumade6, Temitope V Adekanye6, Adeyemi A Okunowo6,7 and Kehinde S Okunade6,7,8
1Department of Obstetrics and Gynaecology, Lagos Island Maternity Hospital, Lagos 102273, Nigeria
2Genome Editing Research Group, Department of Cell Biology and Genetics, University of Lagos, Lagos 101017, Nigeria
3Department of Obstetrics and Gynaecology, Lagos State University Teaching Hospital, Lagos 101233, Nigeria
4Division of Epidemiology and Biostatistics, School of Public Health, University of Witwatersrand, Johannesburg 2193, South Africa
5Department of Obstetrics and Gynaecology, Nnamdi Azikiwe University Teaching Hospital, Nnewi 435101, Nigeria
6Department of Obstetrics and Gynaecology, Lagos University Teaching Hospital, Lagos 102215, Nigeria
7Department of Obstetrics and Gynaecology, College of Medicine, University of Lagos, Lagos 102215, Nigeria
8Center for Clinical Trial Research and Implementation Science, College of Medicine, University of Lagos, Lagos 102215, Nigeria
Abstract
Synopsis: We found a significant increase in serum IL-6 levels of 214.9 ng/mL (95% CI: 60.1–369.7, p = 0.007) among women with cervical cancer (CC) compared to their cancer-free counterparts.
Objectives: Despite growing evidence of the role of interleukin-6 (IL-6) in CC, studies assessing this association among women of sub-Saharan African origin remain limited. This study investigated the association between serum IL-6 and CC and explored the effects of serum IL-6 levels on prognosis in patients with CC in Lagos, Nigeria.
Methods: We conducted a cross-sectional study among women with and without CC in two hospitals. A venous blood sample was collected from each participant for laboratory analysis of serum IL-6 levels. We performed simple and multiple linear regression analyses to compare the unit increase in serum IL-6 levels between study groups while adjusting for relevant confounders.
Results: Our study found a significant unit increase of 214.9 ng/mL (95%CI: 60.1–369.7, p = 0.007) in serum IL-6 levels among women with CC compared to their cancer-free counterparts. The area under the curve of 0.814 demonstrated a good discriminatory ability at an optimal serum IL-6 cut-off value of 365.1 ng/mL. IL-6 levels were significantly elevated in patients with advanced-stage CC compared to those with early-stage disease (156.7 (IQR: 130.4–227.6) versus 324.7 (IQR: 188.5–516.2) ng/mL) and in patients diagnosed with squamous cells carcinoma (SCC) compared to those without SCC (523.9 (IQR: 365.1–682.1) versus 203.6 (IQR: 131.3–334.3) ng/mL).
Conclusion: Our study findings highlight the potential utility of serum IL-6 as a biomarker for CC diagnosis and prognosis. However, further studies are needed to explore the utility of IL-6 as a target for therapeutic intervention and in the treatment monitoring of CC patients.
Keywords: cervical cancer, inflammation, interleukin-6, Nigeria, prognosis
Correspondence to: Kehinde S Okunade
Email: sokunade@unilag.edu.ng
Published: 07/10/2025
Received: 04/04/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cervical cancer (CC) remains a significant public health challenge worldwide [1], with its burden disproportionately affecting low- and middle-income countries [2]. Data indicate that sub-Saharan Africa bears the highest incidence and mortality rates from the disease [1, 3, 4], with Nigeria alone reporting approximately 12,075 new cases and 10,403 deaths annually [2, 5].
The development of CC has been strongly linked to persistent infection with oncogenic human papillomavirus (HPV), the primary etiological agent of the disease and its precursor lesions [6, 7]. However, the progression from HPV infection to invasive cancer is influenced by a complex interplay of environmental, lifestyle and host factors that modulate viral activity [1, 8]. Individual genetic variations in immune mediators play a critical role in susceptibility to neoplasia [9], with increasing evidence suggesting that pro-inflammatory cytokines contribute to CC pathogenesis [10].
Interleukin-6 (IL-6) is a multifunctional cytokine secreted by various cell types, such as macrophages, T cells, fibroblasts and endothelial cells, in response to infections, tissue damage and a range of inflammatory triggers [11, 12]. It is involved in immune regulation, inflammation, haematopoiesis and cancer development and progression [12]. IL-6 has been implicated in tumourigenesis by promoting cell proliferation, angiogenesis and resistance to apoptosis in several cancers [13–15], including CC, and is now considered a critical biomarker and therapeutic target in various diseases, particularly in inflammation-related pathologies and cancer [11, 13–15]. However, despite growing evidence of its role in CC [16–18], studies assessing the association between IL-6 and CC among women of sub-Saharan African origin remain limited. To address this gap and provide valuable insights into the role of IL-6 in CC susceptibility and progression, our study investigated the association between serum IL-6 and CC and then explored the effects of serum IL-6 levels on prognosis in patients with CC in Lagos, Nigeria.
Materials and methods
Study design and setting
This is a cross-sectional study conducted between January and December 2023 at the gynaecological oncology clinics of Lagos State University Teaching Hospital (LASUTH), Ikeja and Lagos Island Maternity Hospital (LIMH), Lagos Island, Lagos, Nigeria. The hospitals serve as public referral centres for other medical facilities in Lagos and its adjoining states. They both run gynaecology, cytology and colposcopy clinics from Monday to Friday of each week, providing evaluation, diagnosis and appropriate treatment for patients with pre-invasive and invasive CC.
Study population
Eligible participants were women aged 18 years or more with histologically confirmed CC (case group) enrolled from the study clinics using the consecutive sampling technique. The comparison group comprised randomly selected, unmatched healthy and cancer-free women attending the gynaecological and cytology clinics during the same study period as the case group. Exclusion criteria included a history of other malignancies, withdrawal of consent during the study and lack of physical and mental capacity precluding understanding of informed consent.
Study procedures
On confirming each participant’s eligibility, informed written consent was obtained upon explanation of the goals and procedures of the study. Information on sociodemographic and clinical characteristics, including participants’ age, parity, marital (married or unmarried) and menopausal (pre- and post-menopausal) status, types of marriage (mono- or polygamous), educational, occupational and socioeconomic status, religion, ethnicity, initiation of sexual activity and age at coitarche, use of alcoholic beverages and oral contraceptive pills, number of lifetime sex partners and International Federation of Gynaecology and Obstetrics (FIGO) stage and histological type of disease, were obtained from each participant, after which about 5 mL of peripheral blood samples were collected through venipuncture into a plain specimen bottle (without anticoagulant), sealed and transported within an hour to the main laboratory at LIMH. The samples were centrifuged to separate the serum, which was then stored frozen at −80°C before batched analysis of serum IL-6 concentrations with Human interleukin-6 ELISA Kit (E0090Hu) (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Sample size calculation
Using the sample size formula for comparing means in Stata, we determined that a total sample size of n = 58 would provide 80% power at a type 1 error rate of 5% with provision for a 20% non-response or data recording error rate. This calculation was based on mean IL-6 values of 23.16 ± 19.57 pg/mL for CC patients and 9.91 ± 9.25 pg/mL for non-CC participants, derived using Hozo’s method [19] from the median IL-6 values reported in the study by Lippitz and Harris [20].
Statistical analysis
Statistical analyses were performed using Stata version 18.0 for Windows (StataCorp LLC, TX, USA). Data were summarised using descriptive statistics with continuous variables presented as mean (standard deviation) for normally distributed data or median (25th—75th centile) for skewed distributions. Serum IL-6 levels between the women with and without CC were compared using the non-parametric Wilcoxon rank sum test. We performed simple (bivariable) and multiple (multivariable) linear regression analyses with a backward conditional approach to compare the unit increase in serum IL-6 levels between the study groups while adjusting for potential confounders such as participants’ age, menopausal status, age at coitarche and the number of lifetime sex partners using coefficient (b) at 95% confidence interval (95%CI). We then performed a receiver operating characteristic (ROC) curve analysis for serum IL-6 to derive an optimal discriminatory cut-off value for serum IL-6 levels that distinguished between CC and non-cancer. Subgroup analyses were also performed to compare the serum IL-6 levels between cancer stage (early versus advanced) and histological type squamous cell carcinoma (SCC versus non-SCC) in women with CC. Statistical significance was reported at p < 0.05.
Statement of ethics
This study was approved by the Health Research Ethics Committee of the LASUTH, the Health Service Commission and the LIMH (approval number LREC/10/1990). We conducted the study ethically per the World Medical Association Declaration of Helsinki. All the study participants read and signed a written informed consent before enrolment, and we ensured strict adherence to the privacy and confidentiality of participants' information during and after conducting the study.
Results
A total of 107 potential participants were screened for eligibility in the study (58 in LASUTH and 49 in LIMH). Of these, 37 (63.8%) were enrolled from LASUTH, while 23 (46.9%) were from LIMH. We included 60 women in the study (30 women diagnosed with CC and 30 cancer-free comparators). The mean age of participants with CC was significantly higher than that of the comparators (52.0 ± 10.0 years versus 42.6 ± 6.7 years, p < 0.001). Similarly, the median age at first sexual intercourse (coitarche) was higher among those with CC compared to the cancer-free comparison group (19.5 years (IQR: 18.0–21.0) versus 17.5 years (IQR: 17.0–18.0), p = 0.005). Similarly, women with CC also reported a higher median number of lifetime sexual partners (5 (IQR: 3–6) versus 3 (IQR: 2–5), p = 0.002). As summarised in Table 1, other sociodemographic characteristics were similar between the groups.
The clinicopathological characteristics of the CC patients (n = 30) are summarised in Table 2. Cervical SCC was the most common histological type of CC (70.0%, n = 21), while the least common was adeno-squamous carcinoma (6.7%, n = 2). Most CC patients presented with advanced disease (36.7%, n = 11).
Table 1. Comparison of sociodemographic characteristics of participants with and without CC (n = 60).
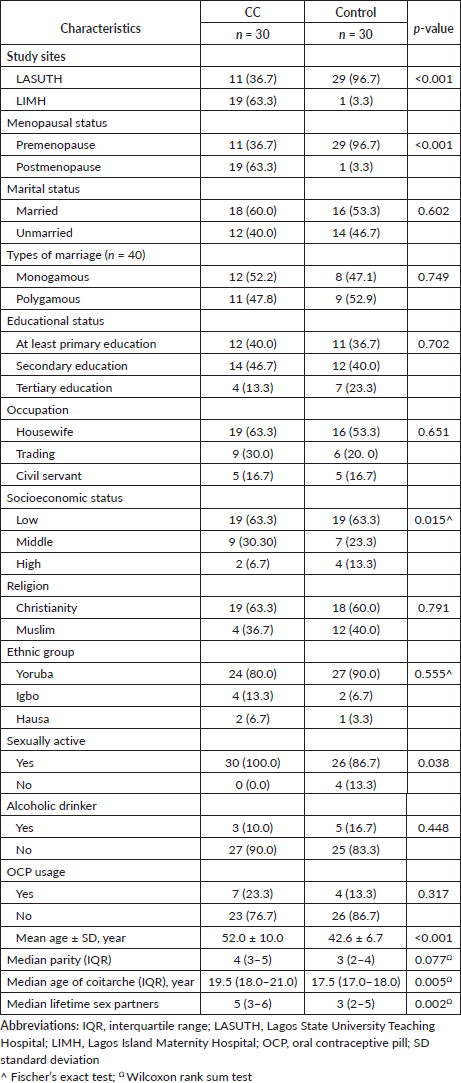
Table 2. Clinicopathological characteristics of CC patients (n = 30).
The median serum IL-6 level was higher in women with CC than in their cancer-free counterparts (476.3 (IQR: 254.9–631.9) versus 192.4 (IQR: 131.0–302.4) ng/mL, p < 0.001) as shown in Figure 1. In the bivariable linear regression analysis performed in Table 2, CC was significantly associated with increased serum IL-6 levels (p < 0.001). As shown in Table 3, after adjusting for potential confounders in the multivariable model, this association remained statistically significant with an estimated unit change of +214.9 ng/mL (95% CI: 60.1–369.7, p = 0.007). Figure 2 shows the ROC curve for serum IL-6, demonstrating its discriminatory ability. The area under the curve (AUC) was 0.814 (95%CI: 0.704–0.925), indicating good diagnostic performance. The optimal serum IL-6 cut-off value for distinguishing between CC patients and their cancer-free counterparts was 365.1 ng/mL, corresponding to a sensitivity of 66.7% and a specificity of 86.7%.
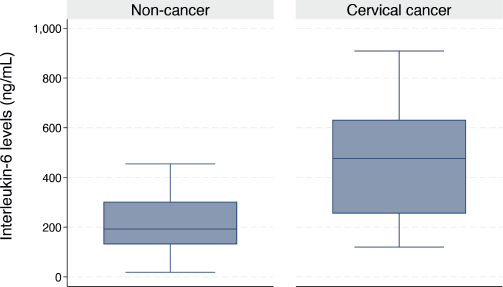
Figure 1. Box plot showing the serum IL-6 levels in women with and without CC (476.3 (IQR: 254.9–631.9) versus 192.4 (IQR: 131.0–302.4) ng/mL, p < 0.001).
Table 3. Linear regression analyses of the association between serum IL-6 levels and CC.
As illustrated in the box plot in Figure 3, the median serum IL-6 level was significantly higher in women with advanced-stage CC compared to those with early-stage disease (324.7 ng/mL (IQR: 188.5–516.2) versus 156.7 ng/mL (IQR: 130.4–227.6), p = 0.015). The median serum IL-6 level was significantly higher in women diagnosed with SCC compared to those without SCC (523.9 ng/mL (IQR: 365.1–682.1) versus 203.6 ng/mL (IQR: 131.3–334.3), p < 0.001) (Figure 4).
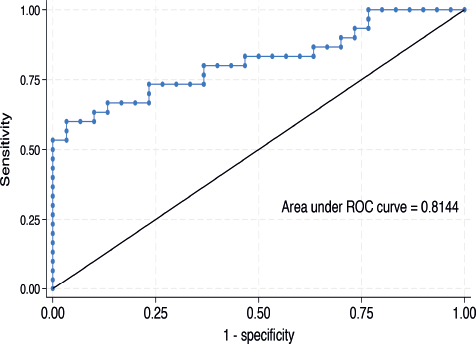
Figure 2. ROC showing an AUC of 0.814 (95%CI 0.704–0.925) at a discriminatory serum IL-6 cut-off value of 365.1 ng/mL.
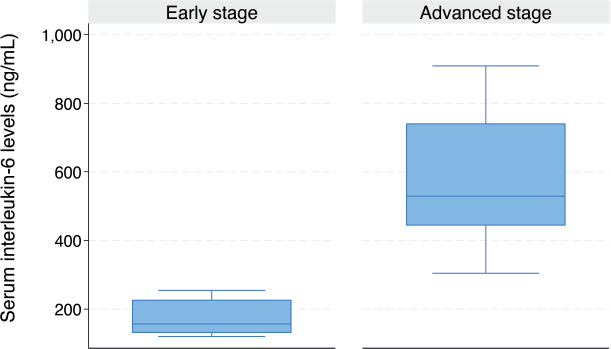
Figure 3. Box plot showing the serum IL-6 levels in women with early and advanced stage CC (156.7 (IQR: 130.4–227.6) versus 324.7 (IQR: 188.5–516.2) ng/mL, p = 0.015).
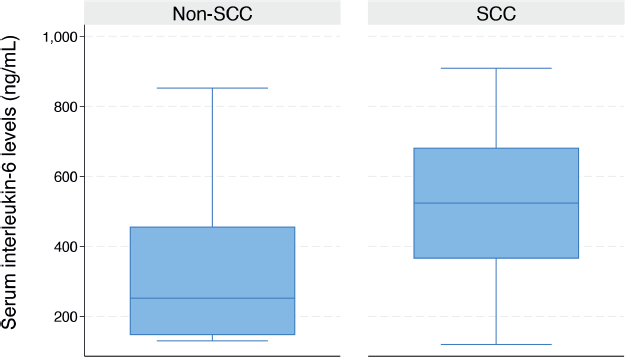
Figure 4. Box plot showing the serum IL-6 levels in women with or without cervical SCC (523.9 (IQR: 365.1–682.1) versus 203.6 (IQR: 131.3–334.3)).
Discussion
Our study evaluated the association between serum IL-6 and CC and explored the effects of serum IL-6 levels on prognosis in patients with CC in Lagos, Nigeria. We reported that serum IL-6 levels were significantly higher in patients with CC compared to cancer-free women, with an optimal serum IL-6 cut-off value of 365.1 ng/mL for distinguishing between CC and cancer-free participants. Further stratification of CC patients revealed that IL-6 levels were significantly elevated in patients with advanced-stage CC compared to those with early-stage disease and in patients diagnosed with SCC compared to those without SCC.
Our study has further provided compelling evidence supporting the association between elevated serum IL-6 levels and CC in corroboration with the findings by Cai et al [16] in Jiangsu, China and Wagh et al [21] in Maharashtra, India. This was even similar to the study by Song et al [18] in Shanghai, China, which reported a significantly higher IL-6 expression in formalin-fixed, paraffin-embedded CC tissues than in adjacent non-tumour tissues examined by immunohistochemistry. This further suggests that IL-6 plays a critical role as a pro-inflammatory cytokine in the tumour microenvironment [22] and its elevated levels in CC patients may reflect ongoing chronic inflammation, which promotes tumour growth, angiogenesis and immune evasion [14]. Persistent infection with high-risk HPV strains, a known driver of cervical carcinogenesis [1], induces inflammatory responses that may contribute to increased IL-6 expression [23]. Furthermore, similarly to previous studies [16, 21], our study demonstrated a high discriminatory ability for serum IL-6, indicating its good diagnostic performance and potential usefulness as a diagnostic adjunct in resource-constrained settings like ours, where access to more advanced diagnostic tools is limited.
As revealed in our study, IL-6 levels were significantly elevated in patients with advanced-stage CC compared to those with early-stage disease. This finding is consistent with previous studies indicating that IL-6 plays a critical role in tumour progression, angiogenesis and immune modulation in CC [15, 16, 18, 20, 21]. The immunosuppressive role of IL-6 contributes to immune evasion by suppressing anti-tumour immune responses, particularly by promoting regulatory T-cell expansion and inhibiting cytotoxic T-cell activity [24]. Additionally, we reported that IL-6 levels were markedly higher in patients diagnosed with SCC than those without SCC, underscoring its role in the pathophysiology of this predominant and inflammation-driven [25] form of CC. These findings reinforce the role of IL-6 as a potential biomarker for CC and highlight its likely relevance in the pathogenesis and progression of the disease [24, 26]. Therefore, given its involvement in inflammation and cancer progression [18, 23], IL-6 could serve as a potential target for therapeutic interventions in CC [11, 14].
The main strength of this study is that it is the first to evaluate the association between serum IL-6 and the risk of CC in Lagos State, Nigeria. However, the study has a few limitations that should be acknowledged. First, we used a cross-sectional study design, which precludes a definitive conclusion of a causal relationship between IL-6 and CC. Second, the sample sizes used in the subgroup analyses may be insufficient to detect significant associations between IL-6 and CC stages and histological types. Therefore, future longitudinal studies with large cohorts and diverse populations are necessary to confirm the findings of our study.
Conclusion
We observed that serum IL-6 levels were comparably higher in patients with CC than in cancer-free women, giving an optimal discriminatory cut-off value of 365.1 ng/mL. Serum IL-6 levels were also significantly higher in patients with advanced-stage CC compared to those with early-stage disease and in those diagnosed with SCC compared to those without SCC. These findings highlight the potential utility of serum IL-6 as a biomarker for CC diagnosis and prognosis. However, further studies are needed to explore IL-6 as a target for therapeutic intervention and assess its use in risk stratification and treatment monitoring in CC patients.
Acknowledgments
The authors thank all the participants, without whom this study would have been impossible.
Conflicts of interest
The authors have no conflicts of interest.
Funding
The authors have received no specific funding for this research. However, the author (KSO) protected time was supported through funding from the National Cancer Institute and Fogarty International Center of the National Institutes of Health under Award Numbers K43TW011930 and D43TW010934 and the Conquer Cancer International Innovation Grant under Project ID 2024IIG-2761200216. The content of this paper is solely the responsibility of the authors. It does not necessarily represent the official views of the National Cancer Institute, Fogarty International Center or the National Institutes of Health and the Conquer Cancer Foundation. The funding agencies were not involved in the study design, manuscript preparation or the decision to submit the manuscript for publication.
Author contributions
Conceptualisation and design: Muisi A Adenekan, Joseph B Minari
Provision of study materials or patients: Muisi A Adenekan, Ayodeji Adefemi, Adebayo Sekumade, Adeyemi A Okunowo, Kehinde S Okunade
Data collection: Muisi A Adenekan, Ayodeji Adefemi, Ayomide I Fayinto, Adebayo Sekumade, Temitope V Adekanye
Data analysis: Gbenga Olorunfemi, Ayomide I Fayinto, Kehinde S Okunade
Funding acquisition: Muisi A Adenekan
Writing—original draft: Muisi A Adenekan, Joseph B Minari, Kehinde S Okunade
Writing—review and editing: Muisi A Adenekan, Joseph B Minari, Adeyemi A Okunowo, Kehinde S Okunade
Supervision: Joseph B Minari, Adeyemi A Okunowo, Kehinde S Okunade
All the authors approved the final version of the manuscript
All authors are accountable for all aspects of the work.
Data availability
The dataset for this study will be made available on reasonable request from the corresponding author (KSO).
References
1. Okunade KS (2020) Human papillomavirus and cervical cancer J Obstet Gynaecol 40 602–608 https://doi.org/10.1080/01443615.2019.1634030 PMCID: 7062568
2. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71 209–249
3. Mwanahamuntu MH, Sahasrabuddhe VV, and Kapambwe S, et al (2011) Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia PLoS Med 8 e1001032 https://doi.org/10.1371/journal.pmed.1001032 PMID: 21610859 PMCID: 3096609
4. Sankaranarayanan R (2006) Overview of cervical cancer in the developing world Int J Gynaecol Obstet 95 Suppl 1 S205–S210
5. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, and Pain A, et al (2015) The global burden of cancer 2013 JAMA Oncol 1 505–527 https://doi.org/10.1001/jamaoncol.2015.0735
6. Ezechi OC, Ostergren PO, and Nwaokorie FO, et al (2014) The burden, distribution and risk factors for cervical oncogenic human papilloma virus infection in HIV positive Nigerian women Virol J 11 5 https://doi.org/10.1186/1743-422X-11-5 PMCID: 3896716
7. Kombe Kombe AJ, Li B, and Zahid A, et al (2021) Epidemiology and burden of human Papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation Front Public Health 8 552028 https://doi.org/10.3389/fpubh.2020.552028 PMID: 33553082 PMCID: 7855977
8. John-Olabode SO, Udenze IC, and Adejimi AA, et al (2025) Association between tumour necrosis factor-a polymorphism and cervical cancer in Lagos State, Nigeria Ecancermedicalscience 19 1845 https://doi.org/10.3332/ecancer.2025.1845 PMID: 40259899 PMCID: 12010130
9. Mehta AM, Mooij M, and Branković I, et al (2017) Cervical carcinogenesis and immune response gene polymorphisms: a review J Immunol Res 2017 1–12 https://doi.org/10.1155/2017/8913860
10. Carrero YN, Callejas DE, and Mosquera JA (2021) In situ immunopathological events in human cervical intraepithelial neoplasia and cervical cancer: review Transl Oncol 14 101058 https://doi.org/10.1016/j.tranon.2021.101058 PMID: 33677234 PMCID: 7937982
11. Aliyu M, Zohora FT, and Anka AU, et al (2022) Interleukin-6 cytokine: an overview of the immune regulation, immune dysregulation, and therapeutic approach Int Immunopharmacol 111 109130 https://doi.org/10.1016/j.intimp.2022.109130 PMID: 35969896
12. Naka T, Nishimoto N, and Kishimoto T (2002) The paradigm of IL-6: from basic science to medicine Arthritis Res 4 S233 https://doi.org/10.1186/ar565 PMID: 12110143 PMCID: 3240141
13. Fisher DT, Appenheimer MM, and Evans SS (2014) The two faces of IL-6 in the tumor microenvironment Semin Immunol 26 38–47 https://doi.org/10.1016/j.smim.2014.01.008 PMID: 24602448 PMCID: 3970580
14. Rašková M, Lacina L, and Kejík Z, et al (2022) The role of IL-6 in cancer cell invasiveness and metastasis—overview and therapeutic opportunities Cells 11 3698 https://doi.org/10.3390/cells11223698
15. Mohamed AH, Ahmed AT, and Al Abdulmonem W, et al (2024) Interleukin-6 serves as a critical factor in various cancer progression and therapy Med Oncol 41 182 https://doi.org/10.1007/s12032-024-02422-5 PMID: 38900329
16. Cai C, Peng X, and Zhang Y (2022) Serum IL-6 level predicts the prognosis and diagnosis in cervical cancer patients Int J Womens Health 14 655–663 https://doi.org/10.2147/IJWH.S347740 PMID: 35547839 PMCID: 9081182
17. Wei LH, Kuo ML, and Chen CA, et al (2001) Interleukin-6 in cervical cancer: the relationship with vascular endothelial growth factor Gynecol Oncol 82 49–56 https://doi.org/10.1006/gyno.2001.6235 PMID: 11426961
18. Song Z, Lin Y, and Ye X, et al (2016) Expression of IL-1α and IL-6 is associated with progression and prognosis of human cerical cancer Med Sci Monit 22 4475–4481 https://doi.org/10.12659/MSM.898569 PMID: 27866212 PMCID: 5120643
19. Hozo SP, Djulbegovic B, and Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample BMC Med Res Methodol 5 13 https://doi.org/10.1186/1471-2288-5-13 PMCID: 1097734
20. Lippitz BE and Harris RA (2016) Cytokine patterns in cancer patients: a review of the correlation between interleukin 6 and prognosis Oncoimmunology 5 e1093722 https://doi.org/10.1080/2162402X.2015.1093722 PMID: 27467926 PMCID: 4910721
21. Wagh P, Kulkarni P, and Kerkar S, et al (2021) Polymorphism of interleukin-6 -174 G/C (rs1800795) & the corresponding interleukin-6 level as a prognostic marker of cervical cancer Indian J Med Res 154 391–398 https://doi.org/10.4103/ijmr.IJMR_1111_19 PMID: 34854425 PMCID: 9131768
22. Masjedi A, Hashemi V, and Hojjat-Farsangi M, et al (2018) The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer Biomed Pharmacother 108 1415–1424 https://doi.org/10.1016/j.biopha.2018.09.177
23. Fernandes JV, De Medeiros Fernandes TAA, and De Azevedo JCV, et al (2015) Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review) Oncol Lett 9 1015–1026 https://doi.org/10.3892/ol.2015.2884 PMCID: 4315066
24. Bent EH, Millán-Barea LR, and Zhuang I, et al (2021) Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy Nat Commun 12 6218 https://doi.org/10.1038/s41467-021-26407-4 PMID: 34711820 PMCID: 8553783
25. Deivendran S, Marzook KH, and Radhakrishna Pillai M (2014) The role of inflammation in cervical cancer Adv Exp Med Biol 816 377–399 https://doi.org/10.1007/978-3-0348-0837-8_15 PMID: 24818731
26. Briukhovetska D, Dörr J, and Endres S, et al (2021) Interleukins in cancer: from biology to therapy Nat Rev Cancer 21 481–499 https://doi.org/10.1038/s41568-021-00363-z PMID: 34083781 PMCID: 8173513






