Germ cell tumours of the testis: 10-year survival data from a tertiary care centre in India
Nidhi Gupta1,a, Kislay Dimri1, Aanchal Arora2, Awadhesh Kumar Pandey1 and Ashok Kumar Attri3
1Department of Radiation Oncology, Government Medical College and Hospital, Chandigarh 160030, India
2Department of Community Medicine and School of Public Health, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh 160012, India">
3Department of General Surgery, Government Medical College and Hospital, Chandigarh 160030, India
ahttps://orcid.org/00000340792631
Abstract
Introduction: Germ cell testicular tumours are rare tumours. The incidence is the lowest in India, leading to limited availability of published Indian data. We report here the 10-year survival data for patients with this curable malignancy.
Material and methods: Record-based analysis was done for testicular germ cell tumours presenting to a tertiary care referral centre in North India during the period from 2010 to 2019. A total of 44 patients were identified who were evaluated for the demographics, treatment modalities and 10-year disease-free survival and overall survival (OS).
Results: Forty five percent of the patients had seminoma, while 55% had nonseminomas. Stages I–III disease was seen 41%, 23%, 36% and 67%, 17%, 17% of nonseminoma and seminoma patients, respectively. Within the seminomas, 89% patients were good risk and 11% were intermediate risk. Within the nonseminoma patients, 81% were good risk, 13% were intermediate risk and 6% were poor risk. At a median follow up of 73.4 months, 5- and 10-year OS were 88% and 77% for seminoma, while 87% and 78% for nonseminomas. The 5- and 10-year progression-free survival was 88% and 76% for seminoma patients, while 83% each for nonseminoma patients. On Cox proportional univariate analysis, none of the prognostic factors were found to be associated with OS.
Conclusion: Our patients presented with a lower metastatic disease burden, minimal violation of the scrotum and upfront orchiectomy in all patients. This resulted in better survival outcomes compared to previous Indian studies. However, the outcomes are inferior as compared to the West. Raising awareness about early diagnosis, treatment safety and curability may further save lives in these young males.
Keywords: testis, seminoma, nonseminoma, survival, germ cell tumour
Correspondence to: Nidhi Gupta
Email: nidhiguptaonco@gmail.com
Published: 30/09/2025
Received: 28/03/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (
Introduction
Testicular cancer is a rare tumour and constitutes less than 1% of all male tumours [1]. India has one of the lowest incidences of testicular carcinoma globally. The age-standardised incidence rate of testicular cancer in India is 0.5 per 100,000 population, while it is 6.7 and 5.6 per 100,000 population for Europe and the United States, respectively [2]. Risk factors associated with testicular tumours include a positive family history or a history of cryptorchidism [3].
The most common testicular tumours are the germ cell tumours, which comprise 95% of all malignant testicular tumours. Germ cell tumours of the testis are of two types- seminoma and nonseminoma [3]. Pure seminomas are more common, slow-growing tumours and present at an early stage as compared to nonseminomas, which are less common, more aggressive and present with advanced stage [3, 4]. The four histological subtypes of nonseminomas are embryonal carcinoma, choriocarcinoma, yolk sac tumour and teratoma [4].
Most commonly, a testicular tumour patient presents with a unilateral testicular mass associated with scrotal pain or back pain. Investigative workup includes a trans-scrotal ultrasound with Doppler. Serum tumour markers, including alpha feto protein, beta human chorionic gonadotropin and lactate dehydrogenase, are assessed as required for diagnosis, staging and prognosis [5]. Histopathological confirmation of the diagnosis of germ cell tumour of the testis is made after radical inguinal orchiectomy. Metastatic workup includes contrast-enhanced computed tomography (CECT) scan of abdomen, pelvis and chest. Positron emission tomography (PET) scan does not have a primary role in staging testicular germ cell tumours [3, 6]. Post orchiectomy tumour markers should be repeated. Persistent or increasing tumour markers indicate the presence of systemic disease [6].
Based on the disease extent as determined clinically, radiologically and postoperative tumour marker levels, patients are categorised into stages and risk groups according to the Union for International Cancer Control [7] and the International Germ Cell Cancer Collaborative Group (IGCCCG) [8]. Further management is diverse, ranging from active surveillance, radiotherapy, chemotherapy, resection or a combination of these. Outcomes for germ cell testicular tumours remain excellent even for advanced stage disease and 5-year overall survival (OS) ranges from 100% for stage I disease to 75%–85% for advanced metastatic disease [9].
In view of the rarity of the tumour in the Asian continent, data for this malignancy from India are rare. Very few Indian studies [10–12] have reported about this rare malignancy. There are gaps in the literature regarding the demographic and clinical picture, treatment modalities utilised and outcomes. Long-term survivals have not been reported. We report the 10-year survival for testicular tumour patients being treated at a tertiary care centre in India.
Material and methods
Study design
This is a retrospective observational study that involves a record-based analysis of all testicular germ cell tumour patients diagnosed and treated at a tertiary care referral centre in North India during the period from 2010 to 2019. Patients who had a histopathological confirmation from the institutional pathology department were included in the analysis. A total of 44 patients with testicular germ cell tumours were identified, who were evaluated for their demographic and clinical profile, while 40 patients who reported for treatment were evaluated for treatment details, recurrence patterns and survival outcomes. Four patients who, were lost to follow up and did not take any further treatment after initial evaluation were excluded from this analysis.
Primary and secondary outcomes
Primary outcomes included (i) evaluation of the 10-year disease-free survival and OS. Secondary outcomes included evaluation of (i) the demographic profile, clinical profile and treatment patterns for various stages of seminoma and nonseminoma patients; (ii) the prognostic factors including age, duration of symptoms, post orchiectomy tumour markers, tumour size, lymph node (LN) involvement, presence of systemic metastatic disease and overall stage.
Treatment details
Patients were staged appropriately with imaging, baseline and post-orchiectomy tumour markers. Patients with advanced disease were risk stratified as per IGCCCG classification. Patients received stage-appropriate adjuvant treatment after high inguinal orchiectomy.
Surveillance protocol included a history and physical examination, measurement of serum tumour markers and CECT abdomen and pelvis, every 3 to 6 months for first year, every 6 months for second year, every 6 to 12 months for third year and annually for fourth and fifth year [13].
The preferred first-line chemotherapy for germ cell testicular tumours consisted of the standard bleomycin/etoposide/cisplatin (BEP) regimen (D1–D5) repeated once in 3 weeks [14, 15]. Bleomycin was administered only after the baseline pulmonary function tests. A modified chemotherapy schedule, as per the institutional protocol was used, where patients with good risk usually received 3 cycles of BEP followed by a 4th cycle of etoposide/cisplatin (EP) only, while patients with intermediate and poor risk received 4 cycles of BEP [14–17]. Prophylactic growth factors were not used routinely.
Radiation therapy (RT) included the irradiation of the retroperitoneal lymph nodes (RPLNs) using the para-aortic field extending superiorly from the bottom of T10 to the lower border of L5 inferiorly. Laterally, the field covered the tips of the transverse processes of the vertebrae using anterio/posterior- posterio/anterior fields. In cases with a history of pelvic surgery or advanced stages dog leg field was used to include the ipsilateral pelvic LNs also [18]. The dose of radiation varied from 20–36 Gy depending on the stage and risk categorisation. Radiation was also used for the treatment of residual RPLN post chemotherapy for patients who refused retro peritoneal lymph node dissection (RPLND).
Patients with a complete response after chemotherapy were kept on follow up. Seminoma patients, with residual tumours >3 cm on fluro-deoxy-glucose-PET scan or computed tomography (CT) scan, were offered RPLND if the biopsy was positive. Nonseminoma patients with residual tumour more than 1 cm on CT scan were offered resection or RPLND.
Statistical analysis
Statistical analysis was done using Statistical Package for Social Sciences version 17 (Chicago, IL, USA). Descriptive statistics were used for demographic, clinical parameters and treatment modalities and were reported as median and percentages. OS and progression free survival (PFS) were estimated according to the Kaplan–Meier method, stratified for seminoma and nonseminoma. Cox proportional hazard method was used to assess the prognostic factors using univariate analysis. A ‘p’ value of <0.05 was considered significant.
Administr ative approval for use of data was obtained from the Principal Investigator of the Hospital Based Cancer Registry, which maintains data on all cancer patients treated in the given health facility.
Results
Demography
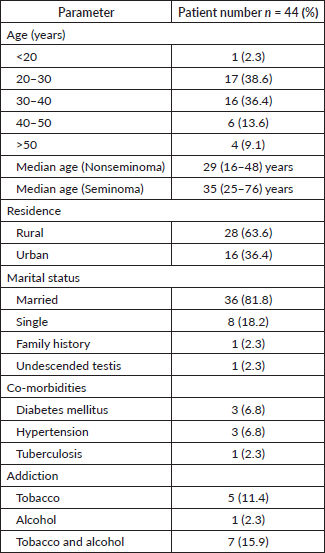
The median age for seminoma and nonseminoma patients was 35 (25–76) years and 29 (16–48) years, respectively. Nearly 82% patients were married, and 64% patients belonged to a rural background. One patient each had a family history of testicular malignancy, and one patient had a history of undescended testis (Table 1).
Clinical profile
The most common presenting symptoms were testicular swelling and pain seen in 71% and 43% patients, respectively. The majority of patients (59%) presented late, more than 3 months after the onset of symptoms. Post-orchiectomy histology was reported as seminoma in 45% patients and nonseminoma in 55% patients (Table 2).
Table 1. Demographic profile.
Stage and risk grouping
Stages I–III disease was seen 41%, 23%, 36% and 67%, 17%, 17% of nonseminoma and seminoma patients, respectively. According to the IGCCCG classification system, 89% seminoma patients were good risk and 11% were intermediate risk. Within the nonseminoma patients, 81% were good risk, 13% were intermediate risk and 6% were poor risk (Table 3).
Treatment
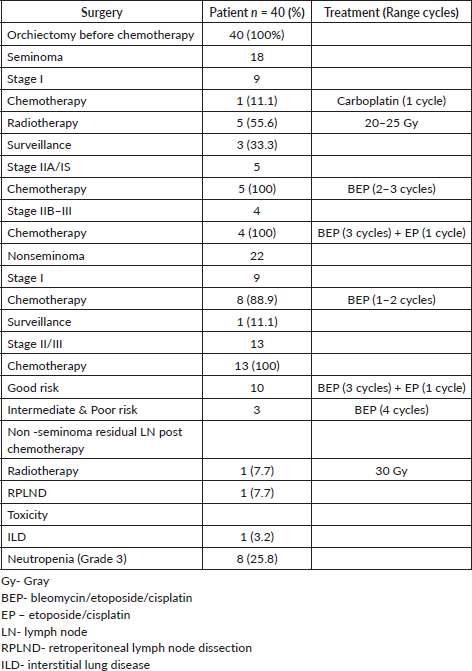
For stage I seminoma patients, 56%, 33% and 11% patients underwent radiotherapy, surveillance and chemotherapy, respectively. All seminoma patients of stage IIA and IS received 2–3 cycles of BEP chemotherapy, while all stage IIB–III seminoma patients, including the one with intermediate risk disease, received 3 cycles of BEP and 1 cycle of EP chemotherapy. For the nonseminoma patients, for stage I, 89% patients received 1–2 cycles of BEP, while only one patient was kept on surveillance. For stages II–III nonseminoma patients, all good-risk patients received 3 cycles of BEP and 1 cycle of EP chemotherapy, while intermediate and poor-risk patients received 4 cycles of BEP. Two nonseminoma patients with post-chemotherapy residual RPLN were offered RPLND; however, only one underwent nerve sparing RPLND and the other patient who refused RPLND was treated with RPLN radiotherapy (30 Gy) (Table 4).
Recurrence patterns and treatment
There were twice the number of recurrences in the nonseminoma patients compared to the seminoma patients. The chemotherapy regimens used for recurrent cases were individualised based on the initial chemotherapy received and consisted of various regimens, including BEP, etoposide/ifosfamide/cisplatin (VIP) and ifosfamide/carboplatin/etoposide (ICE). Two nonseminoma patients also underwent RPLND (Table 5).
Outcomes
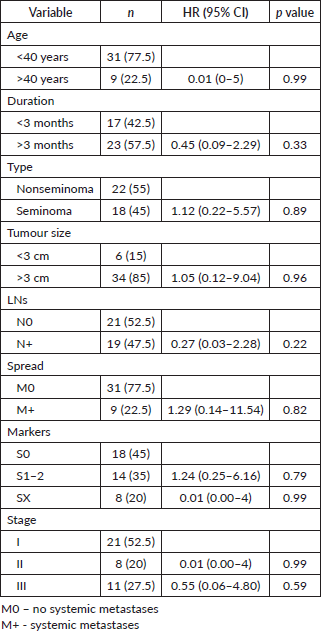
At a median follow up of 73.4 months, the 3-, 5- and 10-year OS were 88%, 88% and 77% for seminoma, while 94%,87% and 78% for nonseminomas (Figure 1). The 5- and 10-year PFS were 88% and 76% for seminoma patients, while 83% each for nonseminoma patients (Figure 2). On Cox proportional univariate analysis, none of the prognostic factors were found to be associated with OS (Table 6).
Table 2. Clinical profile.
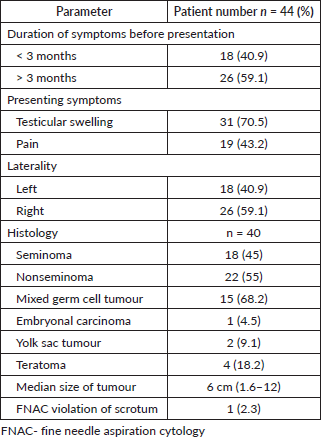
Table 3. Stage and risk grouping.
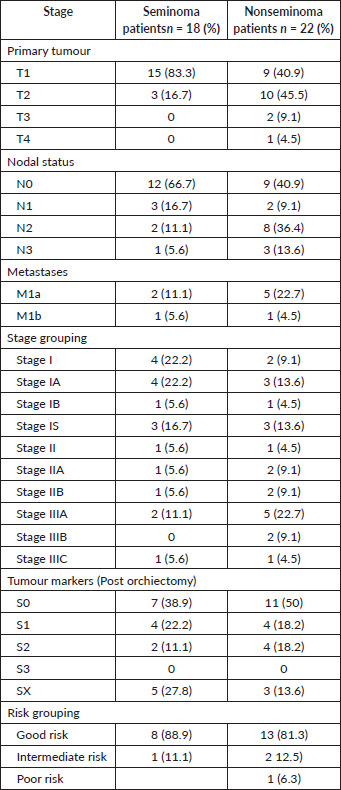
Table 4. Treatment details.
Discussion
Germ cell tumours of the testis are rare but curable tumours of adolescents and young males. The median age at presentation in our series for patients with seminoma and nonseminoma was 35 (25–76) and 29 (16–48) years, respectively, which is similar to the age reported in other Indian studies where seminoma presents in older males as compared to nonseminomas [11, 12, 19]. The majority of patients in our series belong to rural areas, with 59% presenting, 3 months after the onset of symptoms and is an indicator of the geographical barriers associated with delayed presentation and advanced disease [20, 21].
The most common presenting symptom reported was testicular swelling (71%), which is similar to that reported in other Indian studies (71%–89%) [11, 12]. However, 43% patients in our series reported pain at presentation against 27% of Western patients who present with pain [22].
Table 5. Recurrence pattern amongst germ cell testicular tumours.
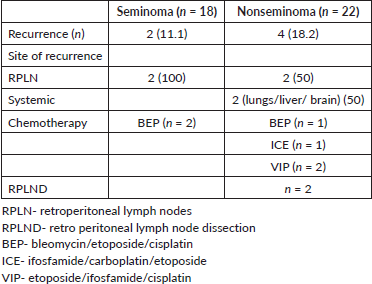
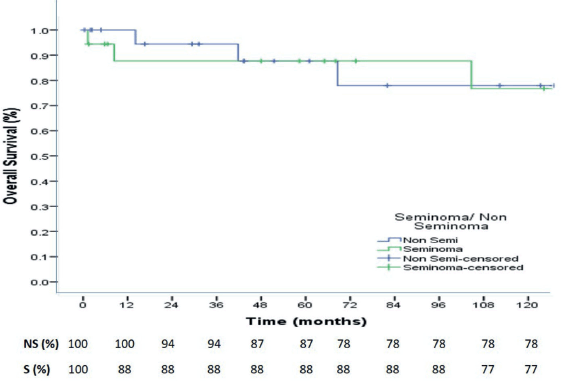
Figure 1. 10-year Overall Survival of seminoma and nonseminoma patients.
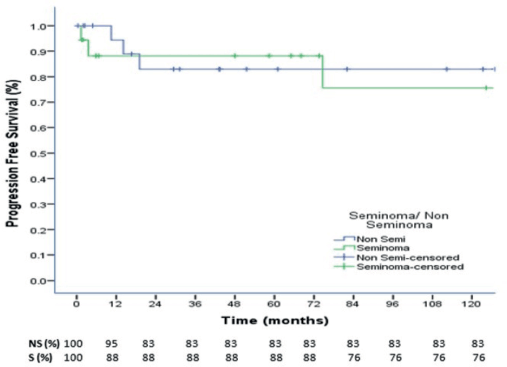
Figure 2. 10-year Progression Free Survival of seminoma and nonseminoma patients.
There is variation in the ratio of seminoma and nonseminoma reported in various Indian studies, with some showing a higher ratio of nonseminoma (70%) to seminoma (30%) [23] and some showing an equal distribution of the two histologies [12]. In our study, seminomas constituted 45% of the cases and nonseminoma constituted 55% of the cases. This contrasts with the Western data, where seminoma remains the predominant histology [3, 24]. Mixed germ cell tumours are the most common subtype of nonseminoma reported globally, which was similar to our study [3, 23].
The majority of Indian studies report that patients in India present in an advanced stage as compared to the West [10–12]. Similar findings were reported in our study with a median size of tumour 6 cm (1.6–12), which is similar to a median size of 6 cm (3.2–12.3) reported by Singh et al [12] and in contrast to a median size of 2.8–3.2 cm reported by a German study [25]. Node-positive disease was seen in 59% and 33% of nonseminomas and seminoma patients, respectively, in our study; within this, N3 disease was seen in 15% of nonseminomas. Randhawa et al [11] reported 25% of N3 disease in seminoma patients. Western data reports N3 disease in less than 5% patients [3, 4, 6]. Overall, for the entire cohort in our study, 53% patients were diagnosed in stage I and 28% were diagnosed in stage III. This stage distribution is better than other studies from India, which report about 50% patients in stage III and 20%–27% patients in stage I [12, 19, 23]. Stage III is reported in less than 5% patients from the West [3, 6]. Nonseminoma patients in the good, intermediate and poor risk categories in our series were 81%, 13% and 6%, respectively. The risk grouping is better than what is reported from other studies from India. Singh et al [12] report that patients with good risk, intermediate risk and high risk were 65.8%, 13.2% and 21.1%, respectively, while Saju et al [23] report 32%, 30% and 38% patients in the good, intermediate and poor risk, respectively, for nonseminoma patients.
All patients were counselled for fertility preservation and sperm banking prior to undergoing orchiectomy, but none consented to the same due to the cost involved [26, 27]. Trans-scrotal biopsies of the testes or trans-scrotal orchiectomy should not be performed because violating the scrotum increases the risk of local or regional recurrence [14]. This is commonly seen in Indian patients (5%–25%) who undergo initial workup in nononcology centres [10, 19, 23]. However, in our analysis, only one patient reported after trans-scrotal fine needle aspiration done outside the institute. All patients in our analysis underwent upfront orchiectomy prior to chemotherapy; however, a significant number of patients in India (13%–21%) present with advanced disease when upfront orchiectomy is not feasible and these patients undergo interval orchiectomy [11, 12, 23].
Table 6. Univariate analysis for OS.
Informed decisions were taken on the management of stage I germ cell tumours based on the risks and benefits associated with each treatment approach [28]. In our analysis, the majority (56%) of stage I seminoma patients received radiotherapy to a dose of 20–25 Gy delivered to para-aortic LNs and one patient received a single cycle of carboplatin. One third of the patients were kept on surveillance. In view of long travel distances, diagnostic costs associated with surveillance and unreliability to adhere to the surveillance protocol, limited patients with good compliance are kept on surveillance [29, 30]. For similar reasons, within nonseminoma stage I patients, only one patient was kept on surveillance and all remaining patients received 1–2 cycles of the BEP regimen. An analysis of more than 5,000 patients with stage I seminoma from various trials reported that the 5-year relapse rate was higher with surveillance (18.6%) compared to RT (4.8% with extended-field RT and 3.6% with para-aortic RT) or chemotherapy (6.1% with 1 cycle of carboplatin and 2.3% with 2 cycles of carboplatin) [31].
All patients of seminoma, stage IIA and IS received 2–3 cycles of BEP, none received radiotherapy. Stage IS pure seminoma with persistent elevation of serum tumour markers following orchiectomy increases the risk of disease outside the retroperitoneum, and hence, systemic therapy should be preferred [32]. A meta-analysis of stage IIA–IIB studies found that in clinical stage IIA with LNs of <2 cm RT and chemotherapy seem to be equally effective at reducing recurrence, while in clinical stage IIB, chemotherapy was more effective [33].
Patients of stage IIB and III seminoma received 3 cycles of BEP and 1 cycle of EP as per the institutional protocol. Three cycles of BEP represent the standard therapy for seminoma patients categorised as good prognosis and four cycles of BEP for intermediate prognosis [34, 35]. The modified approach used at our Institute, helps to maintain the efficacy while reducing the bleomycin-induced cumulative toxicity, in this curable group of young patients with long survival. All seminoma patients post treatment were kept on follow up and did not require post-chemotherapy treatment.
Patients with metastatic nonseminoma stages II and III were either planned for 4 cycles of BEP (intermediate and poor risk) or 3 cycles of BEP and 1 cycle of EP (good risk) as per the risk categorisation. The nonseminoma patients who had post chemotherapy residual masses more than 1 cm on CT scan were offered RPLND; however, patients refusing RPLND were offered par-aortic LN radiation after explaining the risks and benefits. One patient each underwent RPLND and para-aortic LN radiation for post chemotherapy residual disease.
Neutropenia was the predominant toxicity seen in about 25% of the patients receiving chemotherapy, which is similar to other Indian studies (15%–43%) [11, 23]. One patient also developed interstitial lung disease following bleomycin chemotherapy.
Patients mainly recurred in RPLN. Both the recurrences in the seminoma patients and one in nonseminoma patient were seen in patients on surveillance who were salvaged with BEP chemotherapy [36]. Nonseminoma patients with recurrence received different salvage regimens including BEP, VIP and ICE [14, 34]. Two of the nonseminoma patients with RPLN recurrence also underwent RPLND and resection post chemotherapy for residual disease.
At a median follow up of 73.4 months, the 3-, 5- and 10-year OS were 88%, 88% and 77% for seminoma, while 94%, 87% and 78% for nonseminomas. The 5- and 10-year PFS were 88% and 76% for seminoma patients, while 83% each for nonseminoma patients. Neither the OS nor the PFS was significantly different for seminomas and nonseminomas. The OS and PFS reported in our study are comparable to some Indian studies and better than majority of other published Indian studies but overall, our outcomes are inferior to that reported in the western literature which report 5-year OS and PFS for seminomas as 98% and 90%, respectively, while for nonseminomas as 92% and 89%, respectively [37]. In a study from Brazil, for patients with advanced disease (IS, II, III), the 5-year PFS was 88.7% for seminoma and 68.7% for nonseminomas, with 5-year OS of 97.6% and 82.8%, respectively [38].
A study from Southeast Asia reported a 5-year survival rate of 88.9% for germ cell tumours of the testis [39]. From India, Nair et al [19] reported a 4-year OS and PFS for nonseminoma patients as 87.1% and 84.5%, respectively. Saju et al [23] reported a 3-year event-free survival (EFS) and OS of the entire cohort as 73.5% and 80.3%, respectively. The 3-year EFS and OS of the seminoma group were 87.1% and 91.4%, respectively, and the nonseminoma group were 67.4% and 75.3%, respectively [23]. A study from Patna, reported the OS rates for testicular germ cell tumours at 1, 3 and 5 years as 100%, 71.4% and 50.1%, respectively [12]. Another study from north east India reported the 3-year event-free survival and OS as 70.7% and 78.2%, respectively [10]. The better survival rates in our analysis as compared to the majority of Indian studies could be explained by less advanced disease at presentation, low LN burden, patients not undergoing scrotal violation either by trans-scrotal biopsy or trans-scrotal orchiectomy and all patients underwent upfront orchiectomy prior to chemotherapy [40].
On univariate analysis, none of the factors, including age, duration of symptoms, tumour size, post orchiectomy tumour markers and stage, were found to be statistically significant for OS. This was probably due to the small patient number.
Limitations of our study include the retrospective nature and small patient number. However, germ cell testicular tumours are one of the rarest tumours in India, and hence prospective study is not feasible. Data on toxicity were limited due to the retrospective nature. The strength of the study is the long follow up providing a 10-year disease-free and OS, which has not been reported in the previously published Indian studies.
Summary
Testicular tumours are rare in India. Our analysis shows that our patients present in advanced stages with inferior outcomes as compared to the West. However, our outcomes are better than many previous Indian studies as the disease burden in our patients was less than previously reported, trans-scrotal tumour violation was negligible and upfront orchiectomy was performed in all patients.
List of abbreviatio ns
BEP, Bleomycin/etoposide/cisplatin; CT, Computed tomography; EFS, Event free survival; EP, Etoposide/cisplatin; Gy, Gray; ICE, Ifosfamide/carboplatin/etoposide; IGCCCG, International Germ Cell Cancer Collaborative Group; ILD, Interstitial lung disease; OS, Overall survival; PET, Positron emission tomography; PFS, Progression free survival; RPLN, Retro peritoneal lymph nodes; RPLND, Retro Peritoneal lymph node dissection; RT, Radiation therapy; VIP, Etoposide/ifosfamide/cisplatin.
Acknowledgments
None.
Conflicts of interest
None.
Funding
None.
Ethical approval
Administrative approval for use of data was obtained from the Principal Investigator of the Hospital Based Cancer Registry (HBCR), which maintains data on all cancer patients treated in the given health facility. Waiver from the Institutional Ethics Committee was applicable in view of the retrospective nature of the study, which did not involve any patient interaction or intervention.
Author contributions
Conception and design: NG.
Collection and assembly of data: AA and NG.
Data analysis and interpretation: NG, AA, KD.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Accountable for all aspects of the work: All authors.
References
1. Cassell A, Jalloh M, and Ndoye M, et al (2020) Review of testicular tumor: diagnostic approach and management outcome in Africa Res Rep Urol 12 35–42 PMID: 32110551 PMCID: 7035899
2. Shanmugalingam T, Soultati A, and Chowdhury S, et al (2013) Global incidence and outcome of testicular cancer Clin Epidemiol 5 417–427 PMID: 24204171 PMCID: 3804606
3. Oldenburg J, Berney DM, and Bokemeyer C, et al; ESMO Guidelines Committee (2022) Testicular seminoma and non-seminoma: ESMO-EURACAN Clinical Practice guideline for diagnosis, treatment and follow-up Ann Oncol 33 362–375 https://doi.org/10.1016/j.annonc.2022.01.002 PMID: 35065204
4. Katabathina VS, Vargas-Zapata D, and Monge RA, et al (2021) Testicular germ cell tumors: classification, pathologic features, imaging findings, and management Radiographics 41 1698–1716 https://doi.org/10.1148/rg.2021210024 PMID: 34597218
5. Salem M and Gilligan T (2011) Serum tumor markers and their utilization in the management of germ-cell tumors in adult males Expert Rev Anticancer Ther 11 111–114 https://doi.org/10.1586/era.10.219
6. Patrikidou A, Cazzaniga W, and Berney D, et al (2023) European Association of Urology guidelines on testicular cancer: 2023 update Eur Urol 84 289–301 https://doi.org/10.1016/j.eururo.2023.04.010 PMID: 37183161
7. O'Sullivan B, Brierley J, and Byrd D, et al (2017) TNM classification of malignant tumours-towards common understanding and reasonable expectations Lancet Oncol 18 849–851 https://doi.org/10.1016/S1470-2045(17)30438-2 PMID: 28677562 PMCID: 5851445
8. (1997) International Germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ cell cancer collaborative group J Clin Oncol 15 594–603 https://doi.org/10.1200/JCO.1997.15.2.594 PMID: 9053482
9. Kier MG, Lauritsen J, and Mortensen MS, et al (2017) Prognostic factors and treatment results after bleomycin, etoposide, and cisplatin in germ cell cancer: a Ppopulation-based study Eur Urol 71 290–298 https://doi.org/10.1016/j.eururo.2016.09.015
10. Biswas B, Dabkara D, and Ganguly S, et al (2021) Outcome of testicular non-seminomatous germ cell tumours: report from a tertiary cancer centre in eastern India Ecancermedicalscience 11 1204
11. Randhawa AS, Dubey M, and Roy PS, et al (2023) Clinico-epidemiological profile and outcome of testicular germ cell tumors: a retrospective study from a tertiary cancer centre of North-East India Asian Pac J Cancer Care 8 729–734
12. Singh D, Singh P, and Mandal A (2020) Epidemiology and treatment outcomes of testicular germ cell tumor at tertiary care center in Patna, India: a retrospective analysis Asian Pac J Cancer Care 5 45–50
13. van As NJ, Gilbert DC, and Money-Kyrle J, et al (2008) Evidence-based pragmatic guidelines for the follow-up of testicular cancer: optimising the detection of relapse Br J Cancer 98 1894–1902 https://doi.org/10.1038/sj.bjc.6604280 PMID: 18542063 PMCID: 2441965
14. https://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf
15. Saxman SB, Finch D, Gonin R, et al (1998) Long-term follow-up of a phase III study of three versus four cycles of bleomycin, etoposide, and cisplatin in favorable prognosis germ-cell tumors: the Indiana University experience J Clin Oncol 16 702–706 https://doi.org/10.1200/JCO.1998.16.2.702 PMID: 9469360
16. de Wit R, Roberts JT, Wilkinson PM, et al (2001) Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for research and treatment of cancer genitourinary tract cancer Cooperative Group and the Medical Research Council J Clin Oncol 19 1629–1640 https://doi.org/10.1200/JCO.2001.19.6.1629 PMID: 11250991
17. Cary C, Jacob JM, Albany C, et al (2018) Long-term survival of good-risk germ cell tumor patients after postchemotherapy retroperitoneal lymph node dissection: a comparison of BEP x 3 vs. EP x 4 and treating institution Clin Genitourin Cancer 16 e307–e313 https://doi.org/10.1016/j.clgc.2017.10.008
18. Halperin EC, Brady LW, and Perez CA, et al (2013) Perez & Brady's Principles and Practice of Radiation Oncology United States Wolters Kluwer Health
19. Nair LM, Krishna KMJ, and Kumar A, et al (2020) Prognostic factors and outcomes of nonseminomatous germ cell tumours of testis-experience from a tertiary cancer centre in India Ecancermedicalscience 14 1145 PMID: 33343704 PMCID: 7738268
20. Gupta N, Chugh Y and Prinja S (2023) Bridging the cancer care gap and inequities in radiation treatment in India: a narrative review Cancer Res Stat Treat 6 554–561 https://doi.org/10.4103/crst.crst_295_23
21. Halder P, Dixit J, Gupta N, et al (2025) Access to timely cancer treatment initiation in India: extent, determinants and trends Lancet Reg Health - Southeast Asia 32 100514 https://doi.org/10.1016/j.lansea.2024.100514 PMID: 39802701 PMCID: 11722993
22. Germà-Lluch JR, Garcia del Muro X, and Maroto P, et al; Spanish Germ-Cell Cancer Group (GG) (2002) Clinical pattern and therapeutic results achieved in 1490 patients with germ-cell tumours of the testis: the experience of the Spanish Germ-Cell Cancer Group (GG) Eur Urol 42 553–562 https://doi.org/10.1016/S0302-2838(02)00439-6 PMID: 12477650
23. Saju SV, Radhakrishnan V, and Ganesan TS, et al (2019) Factors that impact the outcomes in testicular germ cell tumors in low-middle-income countries Med Oncol 36 28 https://doi.org/10.1007/s12032-019-1252-6 PMID: 30725328
24. Rajpert-De Meyts E, McGlynn KA, and Okamoto K, et al (2016) Testicular germ cell tumours Lancet 387 1762–1774 https://doi.org/10.1016/S0140-6736(15)00991-5
25. Dieckmann KP, Richter-Simonsen H, and Kulejewski M, et al (2018) Testicular germ-cell tumours: a descriptive analysis of clinical characteristics at first presentation Urol Int 100 409–419 https://doi.org/10.1159/000488284 PMID: 29649815 PMCID: 6039091
26. Huyghe E, Matsuda T, and Daudin M, et al (2004) Fertility after testicular cancer treatments: results of a large multicenter study Cancer 100 732–737 https://doi.org/10.1002/cncr.11950 PMID: 14770428
27. Gupta N and Takkar N (2024) Fertility preservation in women undergoing treatment for malignancies: a narrative review Int J Reprod Contracept Obstet Gynecol 13 776–783 https://doi.org/10.18203/2320-1770.ijrcog20240499
28. Dixit J, Gupta N, and Kataki AC, et al (2024) Health related quality of life and its determinants among cancer patients: evidence from 12,148 patients of Indian database Health Qual Life Outcomes 22 26 https://doi.org/10.1186/s12955-024-02227-0
29. Prinja S, Dixit J, and Gupta N, et al (2023) Financial toxicity of cancer treatment in India: towards closing the cancer care gap Front Public Health 11 1065737 https://doi.org/10.3389/fpubh.2023.1065737 PMID: 37404274 PMCID: 10316647
30. Gupta N, Pandey A, and Dimri K, et al (2020) Epidemiological profile of retinoblastoma in North India: implications for primary care and family physicians J Fam Med Prim Care 9 2843–2848 https://doi.org/10.4103/jfmpc.jfmpc_265_20
31. Martin JM, Panzarella T, and Zwahlen DR, et al (2007) Evidence-based guidelines for following stage 1 seminoma Cancer 109 2248–2256 https://doi.org/10.1002/cncr.22674 PMID: 17437287
32. Siddiqui BA, Zhang M, and Pisters LL, et al (2020) Systemic therapy for primary and extragonadal germ cell tumors: prognosis and nuances of treatment Transl Androl Urol 9 S56–S65 https://doi.org/10.21037/tau.2019.09.11 PMID: 32055486 PMCID: 6995840
33. Giannatempo P, Greco T, and Mariani L, et al (2015) Radiotherapy or chemotherapy for clinical stage IIA and IIB seminoma: a systematic review and meta-analysis of patient outcomes Ann Oncol 26 657–668 https://doi.org/10.1093/annonc/mdu447
34. Calabrò F, Albers P, and Bokemeyer C, et al (2012) The contemporary role of chemotherapy for advanced testis cancer: a systematic review of the literature Eur Urol 61 1212–1221 https://doi.org/10.1016/j.eururo.2012.03.038 PMID: 22464311
35. Kawai K and Akaza H (2010) Current status of chemotherapy in risk-adapted management for metastatic testicular germ cell cancer Cancer Sci 101 22–28 https://doi.org/10.1111/j.1349-7006.2009.01373.x
36. Beyer J, Collette L, and Sauvé N, et al; International Germ Cell Cancer Classification Update Consortium (2021) Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-update consortium J Clin Oncol 39 1553–1562 https://doi.org/10.1200/JCO.20.03292 PMID: 33729863 PMCID: 8099394
37. Albany C, Adra N, and Snavely AC, et al (2018) Multidisciplinary clinic approach improves overall survival outcomes of patients with metastatic germ-cell tumors Ann Oncol 29 341–346 https://doi.org/10.1093/annonc/mdx731 PMCID: 6248648
38. Bastos DA, Gongora ABL, and Dzik C, et al (2022) Multicenter database of patients with germ-cell tumors: a Latin American Cooperative Oncology Group registry (LACOG 0515) Clin Genitourin Cancer 21 e104–e113 https://doi.org/10.1016/j.clgc.2022.11.004 PMID: 36509612
39. Tan GH, Azrif M, and Shamsul AS, et al (2011) Clinicopathological features and survival of testicular tumours in a Southeast Asian university hospital: a ten-year review Asian Pac J Cancer Prev 12 2727–30
40. Leman ES and Gonzalgo ML (2010) Prognostic features and markers for testicular cancer management Indian J Urol 26 76–81