CAR-T in relapsed refractory high-grade glioma and glioblastoma – who, what, when and how?
Deevyashali Parekh1, Ansy H Patel1, Areeb Khan1, Eloho Olojakpoke1, Ashay Karpe2, Zoya Peelay3 and Vijay Patil3
1Department of Medicine, SUNY Upstate Medical University, Syracuse, NY 13210, USA
2Department of Medical Oncology, Sunrise Oncology Center, Mumbai 400092, India
3Department of Medical Oncology, Sunact Cancer Institute, Thane 400615, India
Abstract
Recurrent high-grade gliomas have a dismal prognosis. This review article aimed to explore and help answer the questions about which group of patients would benefit from chimeric antigen receptor therapy (CAR-T) cell therapy in this setting, the timing of intervention and the therapeutic efficacy. CAR-T cell therapy involves the extraction of T-cells from patients, genetic modification of these cells to express chimeric antigen receptors on their cell surface, which are selectively targeted towards tumour-expressed antigens and a procedure of immune-depletion followed by re-introducing these engineered CAR-T cells into the host via infusion. Gliomas, particularly glioblastoma, present unique challenges due to their immune-evasive nature, location within the central nervous system and antigenic heterogeneity. Thus, several potential antigenic targets are being explored for CAR-T cell therapy, including B7 homolog 3, Disiloganglioside, Eph-A2, Eph-A3, IL-13Ra2, HER2, EGFRvIII and Matrix metalloproteinase-2.
Keywords: receptors, chimeric antigen, glioblastoma, epidermal growth factor receptor VIII, antigens, neoplasm, CD28 antigens, single-chain antibodies, granzymes, T-lymphocytes, cytokines, matrix metalloproteinase 2, tumour microenvironment, glioma, treatment outcome, central nervous system, cell- and tissue-based therapy
Correspondence to: Vijay Patil
Email: vijaypgi@gmail.com
Published: 30/09/2025
Received: 22/04/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Recurrent high-grade glioma and glioblastoma have a dismal prognosis. No standard of care systemic therapy has been established for these tumours. The National Comprehensive Cancer Network guidelines would suggest the use of Lomustine (CCNU), temozolomide or Procarbazine, CCNU and Vincristine in this situation. The use of Bevacizumab, an antiangiogenic agent, in this situation is described in the literature, with promising results being reported in a phase 2 study [1] (BELOB). However, the agent failed to make its mark in the phase 3 randomised study [2] (EORTC 26101). In this study, the addition of Bevacizumab to CCNU marginally improved the median progression-free survival (PFS) by 2.7 months, but there was no improvement in quality of life or overall survival (OS). Thus, at present, there is a large scope for improvement in the prognosis of these recurrent-refractory high-grade glioma and glioblastoma.
Cellular therapies like Chimeric antigen receptor therapy (CAR-T) have established themselves as the standard of care therapies in CD-19 positive B cell lymphomas and leukaemias and B-cell maturation antigen CAR-T has established itself in Multiple Myeloma. Solid tumour CAR-T has been showing promising results in phase 1 and phase 2 studies. CAR-T innovation enables us to, theoretically, do systemic targeted elimination of cancer cells in a major histocompatibility complex independent manner. However, the brain is considered an immune-privileged organ, and whether CAR-T would be able to have similar efficacy as haematological tumours in this tumour is an open question. Hence, this review article was planned by the authors with the aim of answering the questions of who, when, what and how CAR-T can benefit high-grade glioma or glioblastoma patients from a clinician's perspective.
Methods
The authors performed an advanced PubMed search on 1st March 2025 with the following keywords
- ‘CAR T’[All Fields] AND ‘Glioma’[All Fields]
- ‘CAR T’[All Fields] AND ‘Glioblastoma’[All Fields]
The filter applied in the process was those articles published in the last 5 years. We came across 195 and 233 articles, respectively, with the two keyword searches. Then, manually among these articles, unique articles were selected, subjected to below-mentioned criteria
- Human research
- Case report or phase 1 or phase 2 or phase 3 articles.
We identified 365 unique articles, and these articles were used to answer the questions about CAR-T in whom, when, what and how from the clinician's perspective. The question of who and when was answered by looking at the inclusion and exclusion criteria in the studies. The question of what and how took into account the CAR-T details, its antigen and method of delivery. This is depicted in Figure 1. In addition, the efficacy of these therapies and adverse events were collated. In addition, an algorithm for how to use this knowledge in clinics has also been provided in Figure 2.
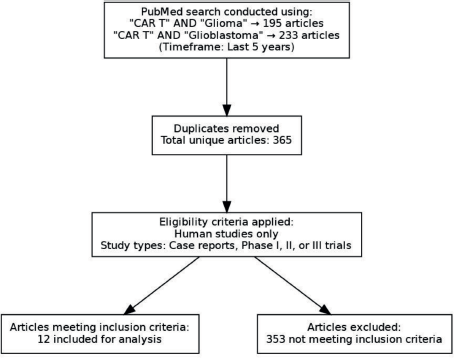
Figure 1. Flowchart of search strategy and article selection.
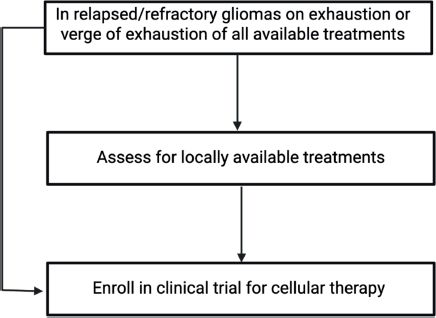
Figure 2. Algorithm for treatment assessment of r/r glioma patients.
Discussion
While our study aims to answer the clinical questions about the patient population, CAR-T selection and timing for CAR-T use in gliomas, it is important to understand the underlying mechanism of how CAR-T therapy works and the specific targets being developed and used in clinical studies.
CAR-T cells are a relatively novel immune-mediated therapy developed in an attempt to overcome factors like dependence on host immunogenicity, immune-escape by tumours, heterogeneity in the tumour microenvironment (TME), leading to reduced efficacy and factors leading to resistance against conventional chemotherapeutic strategies. CAR-T cell therapy involves the extraction of T-cells from patients, genetic modification of these cells to express chimeric antigen receptors (CARs) on their cell surface, which are selectively targeted towards tumour-expressed antigens and a procedure of immune-depletion followed by re-introducing these engineered CAR-T cells into the host via infusion.
CAR-T cells are broadly engineered with three key domains. An extracellular antigen recognition domain, a transmembrane domain responsible for downstream activation and intracellular domains responsible for the induction of the anti-tumour response. The extracellular domain typically consists of a single-chain variable fragment (scFv) that is derived from an antibody against the targeted tumour-specific antigen with the function of selective antigen-recognition. The transmembrane and intracellular domains are responsible for downstream signalling and activation of T-cells, release of cytotoxic granzymes and inflammatory cytokines for the anti-tumour activity. Modifications in subsequent generations of CAR-T cell therapy is generally within these two domains and aim to produce greater longevity of action, amplification of immune effector cells and/or improved therapeutic efficacy.
First-generation CAR-T cells consist of a scFv domain, a transmembrane domain and an intracellular CD3-signaling domain. The first-generation CAR-Ts had a relatively lower duration of action and clinical efficacy. Second-generation CAR-T cells incorporated a costimulatory domain like CD28 OR 4-1BB in an effort to increase the duration of effect. Third-generation CAR-T cells were able to simultaneously incorporate these costimulatory domains, like CD28 and 4-1BB, sometimes OX-40 to further increase the duration of effect. Recently, a fourth generation of CAR-T cells is being worked on, which is being termed an ‘armoured CAR-T cell’ or ‘TRUCK-T’, which supplements a third-generation CAR-T cell with surface-bound cytokines acting as a third signal and serving to modulate the TME or enhance duration of action and efficacy [3]. Recent data in animal models has shown how the addition of these pro-inflammatory cytokines significantly improves the efficacy of CAR-T cells even in high-grade gliomas [4]. Figure 3 depicts CAR-T structure and generations. Figure 4 depicts CAR-T mechanist of action.
Gliomas, including glioblastoma, bring a unique therapeutic challenge. Their location in the ‘immune-protected’ central nervous system (CNS) means that systemic treatments have the added challenge of navigating the blood-cerebrospinal fluid (CSF) barrier and maintaining their efficacy in the CNS. There is a lack of distinct glioma-specific antigens and a wide heterogeneity in their expression, which is a challenge in developing a therapeutic option. It is postulated that certain gliomas, like glioblastoma, can interact with and alter their TME, inducing significant heterogeneity and treatment resistance [5].
Due to the lack of clear-cut targetable antigens in gliomas, several potential antigens have been targeted in clinical trials. These include B7 homolog 3 (B7-H3), Disiloganglioside (GD2), Eph-A2, Eph-A3, IL-13Ra2, HER2, EGFRvIII and Matrix metalloproteinase-2 (MMP-2).
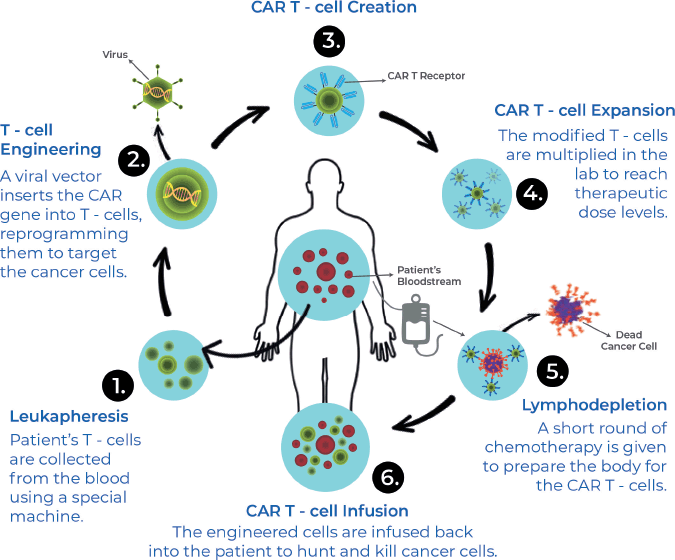
Figure 3. CAR-T therapy production process.
B7 homolog 3
B7-H3, also known as CD276, is a member of the B7 family of immune checkpoint inhibitory molecules. It exerts an inhibitory influence on immunoregulation. It is selectively expressed to a large degree in tumours, like gliomas, and in cells in the TME compared to normal cells in the body. It has also been postulated to play a role in tumour proliferation and treatment resistance [6]. It is expressed in 60%–90% of gliomas in clinical studies [7]. Due to its high degree of expression in gliomas compared to normal brain tissue, it is identified as a potential marker for targeted CAR-T therapy. Pre-clinical studies demonstrated the efficacy of B7-H3 targeting CAR-T cells in vitro in mouse models [8].
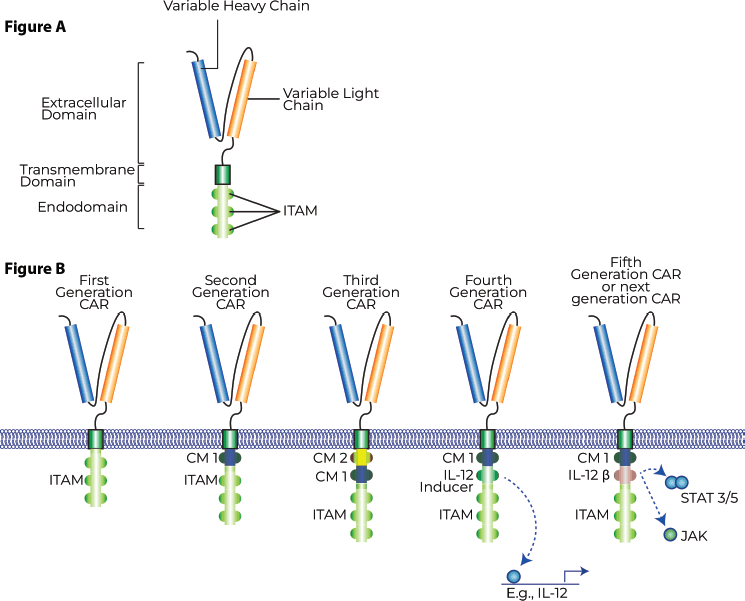
Figure 4 (A): General structure of a chimeric antigen receptor T-cell. (B): Generations of CAR-T cells.
Vitanza et al [9] published updated phase I data in 2025 and included patients with diffuse intrinsic pontine glioma (DIPG) aged 1–26 with a median of 6 years. They had a median OS of 10.7 months (range 0.6–45.8 months) on treatment. Given the aggressive nature of DIPG and its median survival being 8–11 months, a 3-year 40% survival for DIPG patients without a prior progression with B7-H3 CAR-T therapy is remarkable. A case report [10] with B7-H3 CAR-T therapy demonstrated a rapid and dramatic reduction in size of recurrent glioblastoma multiforme (GBM) but this response was short-lived with another recurrence at 50 days after the first infusion.
Disiloganglioside
GD2 is a glycosphingolipid with a sugar chain, found in larger quantities on the cell-surface of CNS tumours, including GBM and DIPG. It is expressed minimally in other CNS tissue. While gangliosides are generally widely expressed in normal tissue, GD-2 specifically is a tumour-associated cell-surface marker and ‘positivity’ for GD2 has been associated with the development of malignant phenotypes, while cell-cycle dysregulation [11]. GD2 positivity has been noted in 50%–90% of malignant gliomas [12]. GD2-targeting CAR-T cells demonstrated efficacy against GBM and DIPG in pre-clinical studies in in-vitro models [13–15].
Phase 1 data by Liu et al [116] with 4SCAR-T targeting GD2 in GD2-positive GBM showed a median OS of 10 months (3–24 months) in 8 evaluable patients. 3 of them achieved a partial response (PR), 1 with stable disease (SD) and 4 had progressive disease (PD). 4 patients were still alive at 12 or more months after receiving the CAR-T infusion with the longest being 24 months at the time of publication. 4 out of the 8 patients had survival of 23 months or longer (3 still alive at time of censoring).
EphA2 and EphA3
Eph receptors comprise the largest group of tyrosine kinase receptors and are divided further into A or B depending on the extracellular domain. They play a role in signal transduction pathways in cells affecting growth, differentiation and migration. EphA2 has been demonstrated to be elevated in 90% of cells of GBM but was not found to be so in normal brain tissue; additionally, it was expressed in a discordant manner to its associated ligand ephrinA1 in GBM, thus possibly demonstrating a role in the pathogenesis of this tumour [17]. Data from Lin et al [18] of a first in-human trial for EphA2-directed CAR-T cells in 3 patients showed 1 SD and 2 PDs with an OS of 86 to 181 days reported.
EphA3 has been shown to be highly expressed in cells implicated in the initiation of gliomas and is postulated to be playing a role in maintaining these cells in a state of low differentiation, which is important in the pathogenesis of cancer [19]. EphA3 CAR-T cells showed efficacy against GBM in preclinical studies [20].
IL-13Ra2
IL-13 is an immunoregulatory cytokine. The IL-13 receptor is associated with the IL-13Ra2 ligand chain, noted to be highly expressed in GBM and very low in normal tissue; it is associated with the mesenchymal subtype and portends a poor prognosis. IL-13Ra2 targeting CAR-T cells differ from most other CARs in that their extracellular antigen recognition domain is a naturally occurring IL-13 ligand rather than the scFv found in other CARs.
An initial off-label use of IL-13Ra2-specific CAR-T cells in GBM was reported by Brown et al [21]. Six patients with unresectable recurrent GBM with a mean age of 56.2 (36–66) received this family of CAR-T cells via intracerebral catheter without any dose-limiting toxicities; however, it was minimally in blood detected at the 10-month mark. Median survival was 2.9 months, with the longest being 11.3 months.
The expanded, phase I trial by Brown et al [22] used IL-13Ra2 CAR-T cells in recurrent, high-grade gliomas in a heavily pre-treated population and with 41 of 58 IDH-wild type. Delivery was either intracranial, intra-tumoural or both. Out of 58 evaluated patients, 35% had Grade 3 or worse toxicities, 29 (50%) patients achieved SD or better, 2 (3.4%) achieved a PR, 1 (1.7%) achieved a CR. The overall cohort had a median OS of 8 months, the population with recurrent GBM had a median OS of 7.7 months. Subset analysis was done stratifying patients by high or low/no CD3 infiltrates in pre-treatment tumour samples. For all recurrent high-grade gliomas, intermediate/high CD3 infiltrates showed a median OS of 11.2 months (10.1–34.7) compared to low/no CD3 median OS of 6.5 months (4.8–9.7) in this study. For recurrent GBM, intermediate/high CD3 had a median OS of 10.3 months (9.2-NA) and low/no CD3 had an OS of 6 months (4.6–8.0). Thus, the higher rates of CD3 positivity in pre-treatment samples correlated to improved outcomes with IL-13Ra2 CAR-T therapy.
Bagley et al [23] have reported interim phase 1 data on using bivalent CAR-T cells targeting EGFR and IL-13Ra2 delivered intrathecally in six patients. All six patients experienced immune effector-cell associated neurotoxicity syndrome (ICANS) and 3 SDs at the time of reporting.
HER2
HER2 is a tyrosine kinase receptor expressed in epidermal tissue across the body but upregulated in tumours, including GBM. An ongoing trial, BrainChild-01 by Vitanza et al [24], reported promising phase I data on HER2 targeting CAR-T therapy in relapsed/refractory CNS tumours like gliomas in the pediatric/young adult population. A landmark study by Ahmed et al [25] with 17 patients demonstrated a median OS of 11.1 months (95% CI, 4.1–27.2 months) from first infusion and 24.5 months (17.2–34.6 months) from diagnosis. 5 of the 17 patients survived for greater than 20 months after infusion. 3 patients were alive without any evidence of progression at 24–29 months.
EGFRvIII
EGFRvIII is a specific variant of EGFR that is selectively found in a proportion of GBM cases and not expressed in normal tissue. This allows EGFRvIII to act as a target for selective CAR-T cells against GBM. However, trials using EGFRvIII CARs did not demonstrate desired efficacy. The reasons for this were likely twofold, first these CARs only targeted this single antigen, which, while selective for GBM, was not as frequently upregulated as other antigenic targets discussed in this review, and second, EGFR-wild type remains the most frequently upregulated variant of EGFR in these tumours. To combat this, EGFRvIII CARs were engineered to secrete T-cell engaging antibody molecules (TEAMs) with action against wild-type EGFR.
Goff et al [26] reported a phase I trial of EGFRvIII targeting CAR-T therapy in patients with recurrent GBM expressing EGFRvIII. 18 patients were included, median PFS was 1.3 months (1.1–1.9 months) and median OS was 6.9 months (2.8–10 months), which did not demonstrate meaningful efficacy.
Bagley et al [27] noted the upregulation of PDL-1 in the TME when using EGFRvIII CAR-T therapy; based on this, a trial of combining EGFRvIII CAR-T therapy with anti-PD1 checkpoint inhibitor pembrolizumab. In this 7-patient study, the median PFS was 5.2 months (90% CI 2.9–6.0 months) and the median OS was 11.8 months (90% CI 9.2–14.2 months).
It was hypothesised that the responses to these CARs may not be as expected, possibly due to the generally higher ratio of wild-type EGFR compared to EGFRvIII. An interesting trial by Choi et al [28] used EGFRvIII targeting CARs in conjunction with TEAMs for action against wild-type EGFR. This 3-patient study demonstrated rapid and strong responses; however, the responses did not last long in 2 out of 3 patients, thus requiring optimisation in durability.
Matrix metalloproteinase-2
MMP-2 acts as a proteolytic enzyme and has a role in breaking down extracellular tissue. It is upregulated in tumours and also plays a role in the development of metastasis. Interestingly, the TME of gliomas modifies MMP-2 in a way that a naturally occurring peptide in the venom of scorpion species Leiurus quinquestriatus, chlorotoxin (CLTX), binds to MMP-2 on the surface of gliomas but not of normal brain tissue. This allows CLTX to act as an antigen recognition domain on the surface of CAR-T cells targeting gliomas. A phase 1 study [29] is recruiting patients at the time of this report.
Results
Key clinical trials for CAR-T in recurrent gliomas are described below. Target, patient demographics and performance status are described in Table 1. Outcome data, including OS and/or PFS, alongside adverse effect data, are included in Table 2.
Table 1. Demographic information for key CAR-T trials in gliomas over the past 5 years.
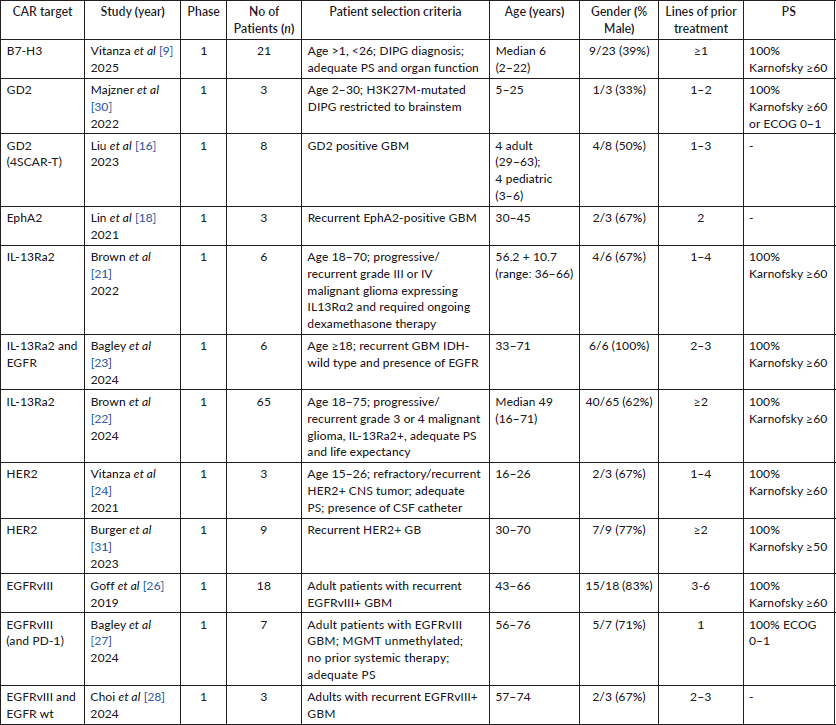
Table 2. Clinical outcome information for key CAR-T trials in gliomas over the past 5 years.
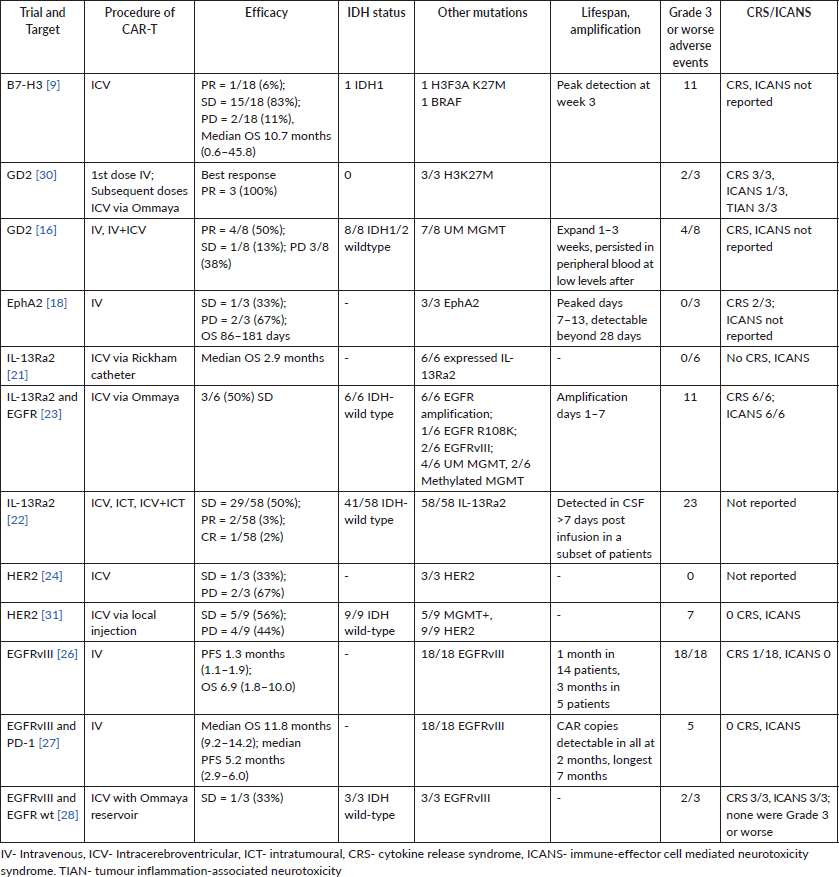
Every trial thus far has been in a population with an adequate performance status, either Karnofsky greater than 50 or Eastern cooperative oncology group (ECOG) 0–1 for recurrent gliomas after first-line treatment. Most trials were able to deliver the engineered CARs intracranially. Lifespan of the CARs was over 3–4 weeks in most studies with some studies demonstrating detectable copies as far as 7 months out. These phase I trials were balancing efficacy versus rates of grade 3 or worse adverse events, CRS and ICANS. Initial glance would suggest that glioma’s demonstrating EGFRvIII and IL-13Ra2 had poorer outcomes as compared to B7H3 and GD2, thus the specific mutation affects outcome even with CAR-T cell therapy. Novel and innovative approaches like bi-target CARs, addition of TEAMs and fourth generation ‘armoured’ CAR-T cell therapy will be needed to tip the scale towards efficacy versus toxicity from this treatment. Phase II/III and long term follow up data will be required to demonstrate survival benefit, toxicities and long-term adverse effects such as secondary primary malignancies, which are beginning to emerge with CAR-T therapy in hematologic malignancies.
Conclusion
Recurrent high-grade gliomas present a unique therapeutic challenge due to their aggressive nature, immune-protected location and ability to interact with the TME. Several antigenic targets are being explored as CAR-T cell therapy, with phase I trials showing relatively promising data in this setting. For patients with an adequate performance status and tumour expressing a targetable cell marker, referral for enrollment in a clinical trial should be considered in the relapsed/refractory setting for gliomas.
List of abbreviations
B7-H3, B7 homolog 3 protein; CAR, chimeric antigen receptor; CAR-T, Chimeric antigen receptor therapy; CCNU, Lomustine, CNS, central nervous system; CLTX, chlorotoxin; CRS, Cytokine release syndrome; DIPG, diffuse intrinsic pontine glioma; ECOG, Eastern cooperative oncology group; GD2, Disiloganglioside; ICANS, Immune effector-cell associated neurotoxicity syndrome; ICT, intratumoural; ICV, Intracerebroventricular; IV, intravenous; MMP-2, Matrix metalloproteinase 2; OS, overall survival; PD, progressive disease; PR, partial response; scFv, single-chain variable fragment; SD, stable disease; TEAMs, T-cell engaging antibody molecules; TME, tumour microenvironment; TIAN, tumour inflammation-associated neurotoxicity.
Conflicts of interest
None.
Funding
No funding.
References
1. Taal W, Oosterkamp HM, and Walenkamp AME, et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial Lancet Oncol 15(9) 943–953 https://doi.org/10.1016/S1470-2045(14)70314-6 PMID: 25035291
2. Wick W, Gorlia T, and Bendszus M, et al (2017) Lomustine and bevacizumab in progressive glioblastoma N Engl J Med 377(20) 1954–1963 https://doi.org/10.1056/NEJMoa1707358 PMID: 29141164
3. Tang L, Pan S, and Wei X, et al (2023) Arming CAR-T cells with cytokines and more: innovations in the fourth-generation CAR-T development Mol Ther 31(11) 3146–3162 https://doi.org/10.1016/j.ymthe.2023.09.021 PMID: 37803832 PMCID: 10638038
4. Look T, Sankowski R, and Bouzereau M, et al (2025) CAR T cells, CAR NK cells, and CAR macrophages exhibit distinct traits in glioma models but are similarly enhanced when combined with cytokines Cell Rep Med 6(2) 101931 https://doi.org/10.1016/j.xcrm.2025.101931 PMID: 39889712 PMCID: 11866521
5. Sharma P, Aaroe A, and Liang J, et al (2023) Tumor microenvironment in glioblastoma: current and emerging concepts Neurooncol Adv 5(1) vdad009 PMID: 36968288 PMCID: 10034917
6. Getu AA, Tigabu A, and Zhou M, et al (2023) New frontiers in immune checkpoint B7-H3 (CD276) research and drug development Mol Cancer 22(1) 43 https://doi.org/10.1186/s12943-023-01751-9 PMID: 36859240 PMCID: 9979440
7. Kontos F, Michelakos T, and Kurokawa T, et al (2021) B7-H3: an attractive target for antibody-based immunotherapy Clin Cancer Res 27(5) 1227–1235 https://doi.org/10.1158/1078-0432.CCR-20-2584 PMCID: 7925343
8. Meeus F, Funeh CN, and Awad RM, et al (2024) Preclinical evaluation of antigen-sensitive B7-H3-targeting nanobody-based CAR-T cells in glioblastoma cautions for on-target, off-tumor toxicity J Immunother Cancer 12(11) PMID: 39562005 Date accessed: 15/03/2025 https://doi.org/10.1136/jitc-2024-009110
9. Vitanza NA, Ronsley R, and Choe M, et al (2025) Intracerebroventricular B7-H3-targeting CAR T cells for diffuse intrinsic pontine glioma: a phase 1 trial Nat Med 31 861–868.
10. Tang X, Wang Y, and Huang J, et al (2021) Administration of B7-H3 targeted chimeric antigen receptor-T cells induce regression of glioblastoma Signal Transduct Target Ther 6(1) 125 https://doi.org/10.1038/s41392-021-00505-7 PMID: 33767145 PMCID: 7994554
11. Nazha B, Inal C, and Owonikoko TK (2020) Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy Front Oncol 10 1000 https://doi.org/10.3389/fonc.2020.01000 PMID: 32733795 PMCID: 7358363
12. Longee DC, Wikstrand CJ, and Månsson JE, et al (1991) Disialoganglioside GD2 in human neuroectodermal tumor cell lines and gliomas Acta Neuropathol 82(1) 45–54 https://doi.org/10.1007/BF00310922 PMID: 1659106
13. Mount CW, Majzner RG, and Sundaresh S, et al (2018) Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas Nat Med 24(5) 572–579 https://doi.org/10.1038/s41591-018-0006-x PMID: 29662203 PMCID: 6214371
14. Gargett T, Ebert LM, and Truong NTH, et al (2022) GD2-targeting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma J Immunother Cancer 10(9) e005187 https://doi.org/10.1136/jitc-2022-005187 PMID: 36167468 PMCID: 9516307
15. Chiavelli C, Prapa M, and Rovesti G, et al (2024) Autologous anti-GD2 CAR T cells efficiently target primary human glioblastoma NPJ Precis Oncol 8(1) 26 https://doi.org/10.1038/s41698-024-00506-z PMID: 38302615 PMCID: 10834575
16. Liu Z, Zhou J, and Yang X, et al (2023) Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma Mol Cancer 22(1) 3 https://doi.org/10.1186/s12943-022-01711-9 PMID: 36617554 PMCID: 9827625
17. Wykosky J, Gibo DM, and Stanton C, et al (2005) EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res 3(10) 541–551 https://doi.org/10.1158/1541-7786.MCR-05-0056 PMID: 16254188
18. Lin Q, Ba T, and Ho J, et al (2021) First-in-human trial of EphA2-redirected CAR T-cells in patients with recurrent glioblastoma: a preliminary report of three cases at the starting dose Front Oncol 11 694941 https://doi.org/10.3389/fonc.2021.694941 PMID: 34235085 PMCID: 8256846
19. Day BW, Stringer BW, and Al-Ejeh F, et al (2013) EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme Cancer Cell 23(2) 238–248 https://doi.org/10.1016/j.ccr.2013.01.007 PMID: 23410976
20. Martins P, D’Souza RCJ, and Skarne N, et al (2024) EphA3 CAR T cells are effective against glioblastoma in preclinical models J Immunother Cancer 12(8) e009403 https://doi.org/10.1136/jitc-2024-009403 PMID: 39111832 PMCID: 11308892
21. Brown CE, Rodriguez A, and Palmer J, et al (2022) Off-the-shelf, steroid-resistant, IL13Rα2-specific CAR T cells for treatment of glioblastoma Neuro Oncol 24(8) 1318–1330 https://doi.org/10.1093/neuonc/noac024 PMID: 35100373 PMCID: 9340633
22. Brown CE, Hibbard JC, and Alizadeh D, et al (2024) Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial Nat Med 30(4) 1001–1012 https://doi.org/10.1038/s41591-024-02875-1 PMID: 38454126 PMCID: 11031404
23. Bagley SJ, Logun M, and Fraietta JA, et al (2024) Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results Nat Med 30(5) 1320–1329 https://doi.org/10.1038/s41591-024-02893-z PMID: 38480922
24. Vitanza NA, Johnson AJ, and Wilson AL, et al (2021) Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis Nat Med 27(9) 1544–1552 https://doi.org/10.1038/s41591-021-01404-8 PMID: 34253928
25. Ahmed N, Brawley V, and Hegde M, et al (2017) HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial JAMA Oncol 3(8) 1094–1101 https://doi.org/10.1001/jamaoncol.2017.0184 PMID: 28426845 PMCID: 5747970
26. Goff SL, Morgan RA, and Yang JC, et al (2019) Pilot trial of adoptive transfer of chimeric antigen receptor–transduced T cells targeting EGFRvIII in patients with glioblastoma J Immunother 42(4) 126–135 https://doi.org/10.1097/CJI.0000000000000260 PMID: 30882547 PMCID: 6691897
27. Bagley SJ, Binder ZA, and Lamrani L, et al (2024) Repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab show no efficacy in glioblastoma: a phase 1 trial Nat Cancer 5(3) 517–531 https://doi.org/10.1038/s43018-023-00709-6 PMID: 38216766
28. Choi BD, Gerstner ER, and Frigault MJ, et al (2024) Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma N Engl J Med 390(14) 1290–1298 https://doi.org/10.1056/NEJMoa2314390 PMID: 38477966 PMCID: 11162836
29. Badie B, Barish ME, and Chaudhry A, et al (2021) A phase 1 study to evaluate chimeric antigen receptor (CAR) T cells incorporating a chlorotoxin tumor-targeting domain for patients with MMP2+ recurrent or progressive glioblastoma (NCT04214392) J Clin Oncol 39(15_suppl) TPS2662 https://doi.org/10.1200/JCO.2021.39.15_suppl.TPS2662
30. Majzner RG, Ramakrishna S, and Yeom KW, et al (2022) GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas Nature 603(7903) 934–941 https://doi.org/10.1038/s41586-022-04489-4 PMID: 35130560 PMCID: 8967714
31. Burger MC, Forster MT, and Romanski A et al (2023) Intracranial injection of natural killer cells engineered with a HER2-targeted chimeric antigen receptor in patients with recurrent glioblastoma Neuro Oncol 25(11) 2058–2071 https://doi.org/10.1093/neuonc/noad087 PMID: 37148198 PMCID: 10628939





