Clinical characteristics and first-line palliative treatment patterns in 3,414 patients with advanced lung cancer in India
Mehak Trikha1†, Vanita Noronha1†, Minit Shah1, Vijay Patil1, Nandini Menon1, Ajaykumar Singh1, Pratik Chandrani2, Omshree Shetty2, Rajiv Kumar Kaushal3, Trupti Pai3, Amit Janu4, Nilendu Purandare5 and Kumar Prabhash1,a
1Department of Medical Oncology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
2Department of Molecular Pathology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
3Department of Pathology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
4Department of Radiodiagnosis, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
5Department of Nuclear Medicine, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
†Have contributed equally for this work
ahttps://orcid.org/0000-0001-8858-5004
Abstract
Lung cancer, particularly non-small-cell lung cancer (NSCLC), is a major global health issue. In India, the complexity of managing this disease is heightened by diverse demographics, varying healthcare access and evolving epidemiological trends influenced by factors such as smoking and advancements in diagnostics. This study explores cancer demographics and first-line palliative treatment options. We conducted a retrospective analysis of 3,414 advanced lung cancer patients planned for palliative systemic therapy at our centre between March 2000 and June 2017. The mean age of the cohort was 56.7 interquartile range (IQR: 21–88) years with a male predominance (71.9%). Histological subtypes included adenocarcinoma (82.9%), squamous cell carcinoma (13.6%) and others (3.5%). Intrathoracic metastases were seen in 40.7% and intrathoracic and extrathoracic in 37.7% of patients. The extrathoracic sites were skeletal (32.5%), liver (14.4%), brain (13.7%) and adrenal gland (9.0%). The baseline Eastern Cooperative Oncology Group Performance status was 0 (8.3%), 1 (58.7%), 2 (20.7%), 3 (11.7%) and 4 in 0.6% of the patients. Epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) positivity rates were lower in smokers. Most patients (92.4%) received the first-line systemic therapy, predominantly platinum-based doublets (67.1%). Tyrosine kinase inhibitors were given to 51.8% of EGFR+, 48% of ALK+ and 54.1% of mutation-negative cases. This study provides crucial insights into lung cancer demographics and treatment patterns at a single tertiary care centre in India, highlighting an increase in female lung cancer cases, steady smoking rates and improved access to genetic testing and targeted therapies.
Keywords: advanced lung cancer, genomic profile, palliative, targeted therapy, epidemiology trend
Correspondence to: Kumar Prabhash
Email: kumarprabhashtmh@gmail.com
Published: 06/03/2025
Received: 28/10/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Lung cancer is one of the most common malignancies globally, contributing to approximately 2.2 million new cases and 1.8 million deaths as of 2020 [1]. The heterogeneity of lung cancers is evident in histological and molecular aspects [2]. Histologically, they are divided into non-small-cell lung cancer (NSCLC, making up 85% of cases) and small-cell lung carcinoma (constituting 15% of cases) [2, 3]. NSCLCs are further classified histologically into adenocarcinoma (ADC), squamous cell carcinoma (SCC) and large-cell carcinoma [2]. Cigarette smoking is the primary cause of lung cancer, followed by additional risk factors such as prolonged exposure to air pollution, occupational carcinogenic exposures and genetic predisposition [4, 5]. Notably, lung cancer incidence in never-smokers and younger individuals is often linked with the ADC subtype’s higher propensity of driver mutation [6].
Most patients with NSCLC present with advanced disease [7, 8]. Traditionally, chemotherapy was the base for the treatment of advanced lung cancer. However, identifying oncogenic driver mutations in NSCLC has revolutionised therapeutic strategies, improving survival outcomes [9, 10]. Genomic studies have identified driver mutations associated with primary lung cancer, including epidermal growth factor receptor (EGFR) [11, 12], anaplastic lymphoma kinase (ALK) [13], ROS1 Proto-Oncogene Tyrosine Kinase Receptor (ROS1) [14] and Serine/Threonine-Protein Kinase BRAF (BRAF) [15]. The advent of targeted therapy drugs has increased the survival rates from a median overall survival of 11 months to a 5-year survival rate of 17.8% [16]. However, challenges persist, particularly in low- and middle-income countries (LMICs), where access to molecular testing and targeted therapy remains limited. In countries like India, molecular diagnostic testing facilities are primarily concentrated in referral hospitals, academic centres or large private laboratories, leading to delayed molecularly profiled cancer diagnoses [8, 17].
Despite being one of the most common cancers, there are limited data on its demography, epidemiology trends and treatment patterns. This study explores the clinical profile, epidemiological trends, access to molecular testing and targeted therapy from a tertiary centre in India.
Material and methods
We performed a retrospective analysis of patients presenting with advanced lung cancer planned for palliative intent systemic therapy at our centre between March 2000 and June 2017. We included the data of patients presenting to the outpatient or in-patient department of the Thoracic Medical Oncology Department with a histologically confirmed diagnosis of lung cancer. They were the patients who were part of the prospectively maintained database and patients willing to participate and provided consent for the same. The management of all patients was based on a multidisciplinary board decision. Data of patients treated with a curative intent were excluded from the analysis.
Patient data were extracted from the Electronic Medical Records (EMR) and the patient’s case file. Patients were telephonically contacted if follow-up data were unavailable in the EMR/case file. The data collected included the patient’s demographic profile, which included age, sex, performance status, comorbidities, smoking habit, tumour characteristics, tumour pathology, site of metastasis, mutation subtype and the first line of treatment received. Patients with NSCLC histology underwent molecular testing with either reverse transcription-polymerase chain reaction (RT-PCR) for EGFR, immunohistochemistry (IHC) or fluorescence in-situ hybridisation (FISH) ALK and ROS1 mutation and next-generation sequencing (NGS) based on feasibility. The study was conducted according to the ethical principles of the Declaration of Helsinki and the Indian Council of Medical Research guidelines. Informed consent was not required due to its retrospective design. The Institutional Ethics Committee has assigned trial number 4,310 for our study. The IEC register number of the trial is 4,310. Informed consent waiver was approved as it is a retrospective study.
Aim and objectives
To explore the clinical characteristics, demographic profile and treatment patterns in advanced non–small-cell lung cancer patients at a tertiary cancer centre in India and to study the change in epidemiological trends, genomic profile and treatment patterns from 2000 to 2017.
Statistics
A formal sample size calculation was not done as this was a retrospective analysis. The data analysis was performed using the Statistical Package for the Social Sciences software (IBM SPSS Statistics for Windows, Version 25.0. IBM Corp., Armonk, NY, USA). Descriptive statistics were used for baseline demographic details using absolute numbers, simple percentages, median, range and IQR. Differences between patient groups were tested using the chi-squared or Fisher’s exact test. In all analyses, p-value <0.05 indicated statistical significance.
Results
Baseline characteristics
This study included a cohort of 3,414 patients with a mean age of 56.7 (IQR: 21–88) years and a male predominance (n = 2,455, 71.9%). In this cohort, 1,765 (51.7%) patients were smokers. ADC was the most common histological subtype seen in 2,830 (82.9%) patients, followed by SCC in 464 (13.6%) and others in 120 (3.5%) patients. The baseline Eastern Cooperative Oncology Group Performance Status (ECOG-PS) was 0 in 284 (8.3%), 1 in 2,004 (58.4%), 2 in 706 (20.7%), 3 in 400 (11.7%) and 4 in 20 (0.6%) patients. The most common sites of metastasis were intrathoracic in 1,141 (40.7%) and intrathoracic and extrathoracic in 1,288 (37.2%) patients. The most common extrathoracic areas were the skeletal (32.5%), followed by the liver (14.4%), brain (13.7%) and adrenal gland (9.0%) (Table 1).
Of the 3,294 patients with NSCLC, driver mutation testing of EGFR by RT-PCR alone or as part of NGS was performed in 2,394 (72.6%) patients. EGFR mutations were observed in 607 (24.1%) patients (Table 2). ALK mutation testing by FISH/IHC was done in 2,200 (66.7%) patients, of whom 272 (12.4%) were positive.
Epidemiological trend
Throughout the study period, the mean age of patients diagnosed with lung cancer was consistent (mean age: 56 years). There was an increase in the proportion of female patients with lung cancer, rising from 28.4% in 2010 to 40.2% in >2016, while the male distribution remained constant. The percentage of smokers declined from 59.4% in 2010 to 44.2% in 2016. Notably, ADC histology showcased a substantial 69.8% increase compared to SCC over the entire study duration. The utilization of molecular testing for driver mutations via RTPCR/IHC/NGS witnessed a rise from 68.1% in 2010 to 78.2% in the period >2016 (Figure 1i–iv).
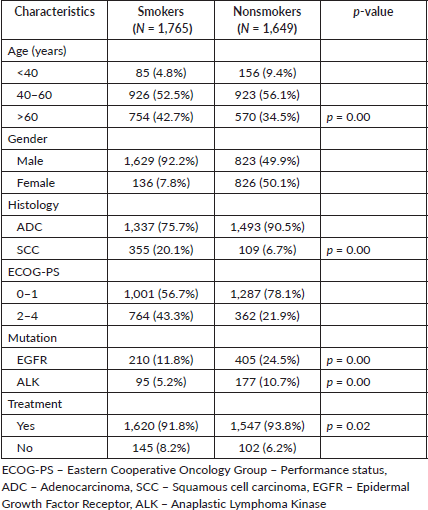
Comparing smokers with nonsmokers, we observed a higher smoking incidence in the >60-year-old age group (42.7% versus 34.5%) with a marked male predominance (92.3%). Smokers displayed a greater incidence of SCC (19.7% versus 6.6%), although ADC histology was more prevalent in both groups. Smokers exhibited significantly lower EGFR and ALK positivity rates (11.8% versus 24.5% and 5.3% versus 10.7%, respectively) (Table 3).
Treatment pattern
First-line systemic therapy was initiated in 3,256 (92.4%) patients. The most common chemotherapy was platinum-based doublet in 2,118 (67.1%) patients. The most common regimen was pemetrexed platinum in 1,471 (46.6%) patients. Tyrosine kinase inhibitors (TKIs) were used as the first-line treatment in 973 (29.5%) NSCLC patients. The 319 (51.8%) EGFR-positive and 127 (48%) ALK+ patients received first-line TKIs. Their usage increased throughout the study. EGFR inhibitor usage in EGFR-positive patients increased from 5.7% to 58.1%, and ALK inhibitor usage in EGFR-positive patients increased from 30.2% in 2011 to 62.1% at >2016. The 527 (54.1%) patients received TKI on compassionate grounds in patients with a driver mutation-negative status (Figure 1v and vi). Four patients with brain metastases underwent surgery, 35 (7.4%) underwent stereotactic radiosurgery and 432 (91.7%) underwent whole brain radiotherapy.
Table 1. Baseline characteristics.
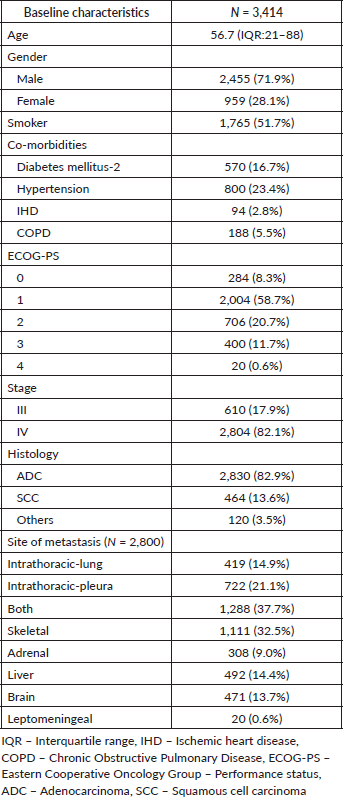
Table 2. Mutation analysis.
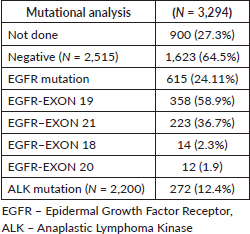
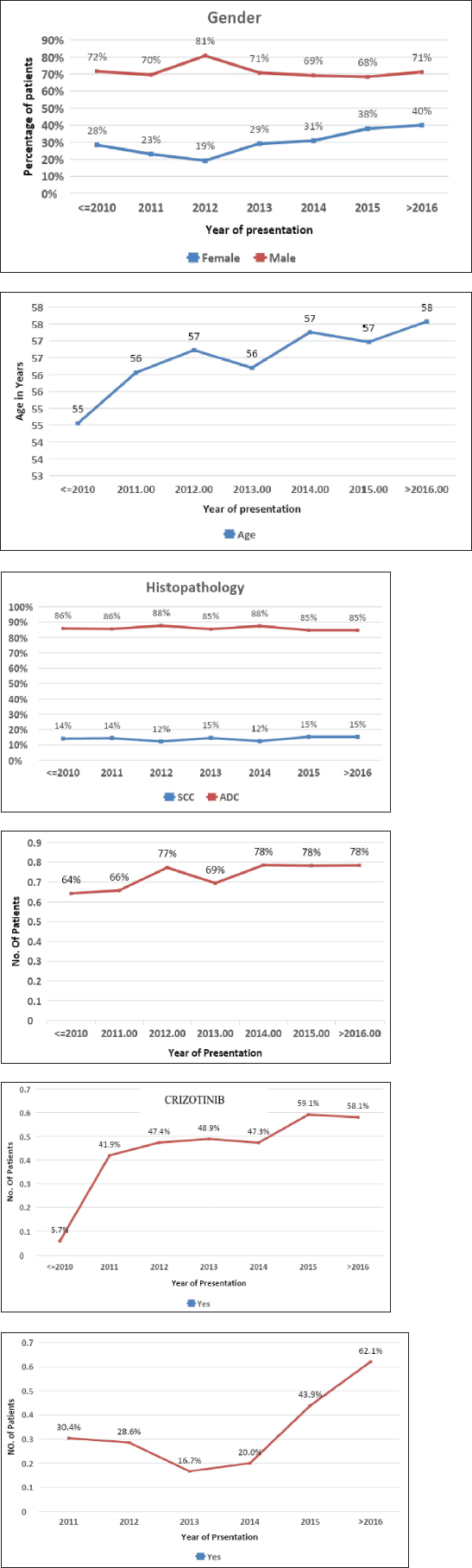
Figure 1. Epidemiological trends. (i): Epidemiology trend in gender (female – 28.4% in <2010 to 40.2% in >2016). (ii): Epidemiological trend in age (stable mean age – 57 years). (iii): Epidemiological trends in histology (ADC 68.6% higher incidence compared to SCC through the study period). (iv): Epidemiological trend in genomic testing (68.1% in 2010 to 78.2% at >2016). (v): Epidemiological trend in use of EGFR TKIs (5.7% in <2010 to 58.1 in >2016). (vi): Epidemiological trend in use of crizotinib (30.4% in 2011 to 62.10% at >2016).
Table 3. Comparison of characteristics between smokers and nonsmokers with lung cancer.
Discussion
To our understanding, this study represents the most extensive single-centre experience examining the demographic profile, treatment patterns and epidemiological trends in the first-line management of patients with advanced non–small-cell lung cancer. The mean age of our cohort was 56 years, consistent with earlier studies conducted in India [18–22]. Yet, intriguingly, it diverges from the age groups reported in Western literature, which is a decade higher [19–25]. The higher number of male lung cancer patients in our study aligns with the Indian population but exceeds the male dominance seen in Western studies. This divergence can be attributed to the higher prevalence of smoking among Western women, which is a well-established risk factor for NSCLC. The prevalence of smoking in our cohort (51.7%) was in agreement with a previous study by Noronha et al [6, 35] but significantly lower than the rates observed in Western countries (80%–90%) [23, 24, 26]. The stability in smoking trends throughout the study period suggests the involvement of other contributory factors in lung cancer aetiology, such as passive smoking, air pollution and indoor smoke exposure, which are more prevalent in the rural regions of India [27, 28]. Throughout the study period, the incidence of ADC histology prevailed over SCC, reflecting the evolving global patterns of NSCLC. This shift can be attributed to the changing dynamics of smoking habits, an increasing incidence of lung cancer among women and improved accuracy in histopathological reporting.
The study revealed a significantly higher prevalence of smoking in the older population (>60 years old), with 42.7% of patients being smokers, in contrast to 34% of nonsmokers. This observation aligns with established trends, suggesting that there is a higher likelihood of smoking in older patients [6]. In addition, the high prevalence of male smokers (92.3%) highlights the well-known gender gap in smoking habits, where men typically smoke more than women [6]. One notable finding was the discrepancy in histological subtypes between smokers and nonsmokers. Smokers exhibited a substantially higher percentage of SCC, with 19.7% of smokers having SCC compared with 6.6% of nonsmokers. This finding was similar to that found in the literature for SCC [19]. Conversely, ADC was the most prevalent histology in both groups. This study also revealed differences in the molecular profiles of smokers and nonsmokers with NSCLC. Smokers exhibited significantly lower rates of EGFR and ALK positivity, with 11.8% of smokers having EGFR mutations compared with 24.5% of nonsmokers and 5.3% of smokers with ALK mutations compared with 10.7% of nonsmokers. These findings reflect the evolving understanding of the impact of smoking on molecular alterations in NSCLC. EGFR, which has been strongly associated with nonsmoking status, particularly in Asian populations [20], has a lower EGFR mutation rate among smokers, which concurs with this known pattern, suggesting that other factors, such as smoking-related mutational burden, may influence the genetic makeup of NSCLC in smokers. Conversely, ALK rearrangements have been associated with younger age and nonsmoking status [21]. The lower prevalence of ALK mutations among smokers could be attributed to distinct mutational profiles associated with smoking-related carcinogenesis.
The proportion of patients with good performance status (ECOG 0–1) at the time of presentation (67.0%) and those with poor performance status (ECOG 2–4, 33.0%) was comparable to that of the Western population [29]. This shift indicates an evolving healthcare landscape and emphasises the need for timely diagnosis and intervention. The study demonstrated a genetic testing rate of 76.3%, revealing a consistent increase from 64% in 2010 to 78% in 2016, mirroring trends observed in Western populations [30]. The introduction of EGFR testing by RT-PCR in 2005, ALK testing by FISH in 2011 and NGS in 2019 reflects the progressive integration of advanced molecular diagnostics at the study centre. Among the tested cohorts, the EGFR positivity rate of 24.1% aligns with findings from Indian studies but surpasses that of Western populations [31–37]. In contrast, the ALK positivity rate of 12.4% exceeds that of the Western population (5%) and ranges from 1.5% to 7.6% in Indian individuals [38–40]. However, it is crucial to acknowledge that testing for ALK mutations was not uniform throughout the study period, which may have affected the observed rate. Moreover, other mutations (mesenchymal–epithelial transition factor) and ROS were underrepresented due to comprehensive panel testing initiated only in EGFR/ALK-negative patients from 2015 onwards. Regarding treatment, 92.4% of the patients initiated first-line systemic therapy, including chemotherapy and targeted therapy reflecting high treatment rates. However, the inability of 158 (4.6%) patients to commence therapy due to poor performance status (1.7%) or logistic challenges (2.9%) highlights the need for targeted interventions to address these barriers, particularly logistical support.
One hundred fifty-eight (4.6%) patients did not receive first-line systemic therapy at our centre; 59 (1.7%) were due to poor performance status and 99 (2.9%) were due to logistic reasons.
Notably, 51.8% of EGFR-positive patients were exposed to TKIs as first-line treatment, albeit lower than the reported real-world data from the USA (72.8%) during a similar period [41]. For ALK-positive patients, 48% received Crizotinib as first-line therapy, aligning with the American literature and exceeding the rates reported in a European study [42, 43]. The increased utilization of Crizotinib in our population is attributed to the amplified support from various governmental and nongovernmental organizations in terms of providing accessibility to the drug.
Over the study period, the increased EGFR and ALK inhibitor usage underscores evolving strategies to enhance access to targeted therapy, including government and private initiatives. Over 50% of patients were administered TKIs on compassionate grounds, considering their compromised baseline performance status or a strong suspicion of mutation positivity based on medical history. Survival advantage in this group of patients has been shown in a study by Shah et al [44]. This opens doors for LMICs with limited resources. In addition, although 3,256 (92.4%) patients commenced first-line systemic therapy, 73 (2.2%) patients were lost to follow-up after initiation, prior to response evaluation. Consequently, the extent of therapy administered to these individuals remains uncertain. This illustrates the challenges associated with ensuring continuity of care, which may adversely affect outcomes, and highlights the necessity for robust patient tracking mechanisms. Furthermore, this attrition underscores the critical need to address nonclinical determinants such as accessibility and adherence to follow-up to enhance treatment delivery and improve outcomes.
Limitations
A survival study could not be conducted due to a significant loss of follow-up data due to logistical and financial constraints.
Conclusion
This study contributes significantly to the comprehensive understanding of demographic profiles, epidemiological trends and access to targeted therapy within a single centre. Our centre’s trajectory appears to align with global histopathological trends in lung cancer. Notably, there was an increase in the proportion of female lung cancer cases, whereas rates of smoking and mean age at diagnosis exhibited no significant changes over time. The expansion of the healthcare sector is evident with the increased use of genetic testing and enhanced patient access to targeted therapies. However, additional efforts are required to address barriers to continuity of care, including follow-up adherence and accessibility, to optimise treatment outcomes in lung cancer.
Acknowledgment
We are grateful to the entire thoracic disease management group for their active contribution to patients’ management and patients and their families for their trust and support.
Conflicts of interest
None.
Funding
None.
Institutional review
The study was conducted according to the ethical principles of the Declaration of Helsinki and the Indian Council of Medical Research (ICMR) guidelines. Informed consent was not required due to its retrospective design and waiver for the same was approved by the ethics committee. The Institutional Ethics Committee (IEC) of Tata Memorial Hospital approved the study.
Author contributions
Mehak Trikha: Conceptualization, methodology, resources, and project administration; writing - original draft preparation and writing: review and editing.
Vanita Noronha: Methodology and investigation; data curation; formal analysis; writing - original draft preparation and writing - Review and Editing.
Minit Shah: Resources, project administration and writing - review and editing.
Vijay Patil: Data curation, methodology and supervision.
Nandini Menon: Data curation and writing: review and editing.
Ajaykumar Singh: Data curation and writing - review and editing.
Prateek Chandrani: Data curation and resources.
Omshree Shetty: Resources, project administration and supervision.
Rajiv Kumar: Resources, project administration and supervision.
Trupti Pai: Resources and writing: review and editing.
Amit Janu: Supervision and resources.
Nilendu Purandare: Resources and writing: review and editing.
Kumar Prabhash: Writing: review and editing; conceptualization, methodology, project administration and supervision.
Data availability statement
All data underlying the results are available as a part of the article, and no additional source data are required.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Inamura K (2017) Lung cancer: understanding its molecular pathology and the 2015 WHO classification Front Oncol 7 193 https://doi.org/10.3389/fonc.2017.00193 PMID: 28894699 PMCID: 5581350
3. Thai AA, Solomon BJ, and Sequist LV, et al (2021) Lung cancer Lancet 398 535–554 https://doi.org/10.1016/S0140-6736(21)00312-3 PMID: 34273294
4. Dubin S and Griffin D (2020) Lung cancer in nonsmokers Mo Med 117 375–379 PMID: 32848276 PMCID: 7431055
5. Herbst RS, Morgensztern D, and Boshoff C (2018) The biology and management of non-small cell lung cancer Nature 553 446–454 https://doi.org/10.1038/nature25183 PMID: 29364287
6. Noronha V, Choughule A, and Patil VM, et al (2017) Epidermal growth factor receptor exon 20 mutation in lung cancer: types, incidence, clinical features, and impact on treatment Onco Targets Ther 10 2903–2908 https://doi.org/10.2147/OTT.S133245 PMCID: 5476719
7. Singh AN, Aggarwal D, and Gupta D, et al (2012) Quantified smoking status and non-small cell lung cancer stage at presentation: analysis of a North Indian cohort and a systematic review of the literature J Thorac Dis 4(2012) 474–484 PMID: 23050111 PMCID: 3461078
8. Mohan A, Garg A, and Gupta A, et al (2020) Clinical profile of lung cancer in North India: a 10-year analysis of 1862 patients from a tertiary care center Lung India 37 190–197 https://doi.org/10.4103/lungindia.lungindia_333_19 PMID: 32367839 PMCID: 7353932
9. Rotow J and Bivona TG (2017) Understanding and targeting resistance mechanisms in NSCLC Nat Rev Cancer 17 637–658 https://doi.org/10.1038/nrc.2017.84 PMID: 29068003
10. Prabhash K, Vora A, and Limaye S, et al (2021) Treatment of advanced non-small-cell lung cancer: first line, maintenance, and second line– Indian consensus statement update(Under the aegis of Lung Cancer Consortium Asia, Indian Cooperative Oncology Network, Indian Society of Medical and Pediatric Oncology, Molecular Oncology Society, and Association of Physicians of India) Cancer Res Stat Treat 4 279–314 https://doi.org/10.4103/crst.crst_61_21
11. Sharma M, Basu DA, and Nathany S, et al (2022) A narrative review of the role of common EGFR mutations in pathogenesis and treatment of non-small-cell lung carcinoma Cancer Res Stat Treat 5 507–518 https://doi.org/10.4103/crst.crst_222_22
12. Rajendra A, Noronha V, and Joshi A, et al (2019) Epidermal growth factor receptor-mutated non-small-cell lung cancer: a primer on contemporary management Cancer Res Stat Treat 2 36–53 https://doi.org/10.4103/CRST.CRST_51_19
13. Nathany S, Sharma M, and Batra U (2023) ALK-driven NSCLC: a narrative review – part I Cancer Res Stat Treat 6 272–278 https://doi.org/10.4103/crst.crst_75_23
14. Nathany S, Batra U, and Sachdeva R, et al (2022) ROS1 in non-small-cell lung carcinoma: a narrative review Cancer Res Stat Treat 5 692–700 https://doi.org/10.4103/crst.crst_322_22
15. Sharma M, Nathany S, and Batra U (2021) BRAF in lung cancer: a narrative review Cancer Res Stat Treat 4 328–334 https://doi.org/10.4103/CRST.CRST_85_21
16. Parikh PM (2012) Lung Cancer Monograph 2012 (ICP, API)
17. Singh N, Agrawal S, and Jiwnani S, et al (2021) Lung cancer in India J Thorac Oncol 16 1250–1266 https://doi.org/10.1016/j.jtho.2021.02.004 PMID: 34304854
18. Jain J, Chinta D, and Jayaraman UB, et al (2019) Determination of ROS1 positivity by immunohistochemistry in a multicentric cohort of 426 non-small-cell lung cancer cases in India Cancer Res Stat Trest 2 16–20 https://doi.org/10.4103/CRST.CRST_12_19
19. Prasad R, James P, and Kesarwani V, et al (2004) Clinicopathological study of bronchogenic carcinoma Respirology 9 557–560 https://doi.org/10.1111/j.1440-1843.2004.00600.x PMID: 15612970
20. Dey A, Biswas D, and Saha SK, et al (2012) Comparison study of the clinicopathological profile of primary lung cancer cases: an Eastern India experience Indian J Cancer 49 89–95 https://doi.org/10.4103/0019-509X.98930 PMID: 22842174
21. Mandal SK, Singh TT, and Sharma TD, et al (2013) Clinic-pathology of lung cancer in a regional cancer center in Northeastern India Asian Pac J Cancer Prev 14 7277–7281 https://doi.org/10.7314/APJCP.2013.14.12.7277
22. Kaur H, Sehgal IS, and Bal A, et al (2017) Evolving epidemiology of lung cancer in India: reducing non-small-cell lung cancer-not otherwise specified and quantifying tobacco smoke exposure are key Indian J Cancer 54 285–290 https://doi.org/10.4103/ijc.IJC_597_16 PMID: 29199707
23. Gadgeel SM, Ramalingam S, and Cummings G, et al (1999) Lung cancer in patients 50 years of age: the experience of an academic multidisciplinary program Chest 115 1232–1236 https://doi.org/10.1378/chest.115.5.1232 PMID: 10334132
24. Fu JB, Kau TY, and Severson RK, et al (2005) Lung cancer in women: analysis of the national surveillance, epidemiology, and end results database Chest 127 768–777 https://doi.org/10.1378/chest.127.3.768 PMID: 15764756
25. Stewart SL, Cardinez CJ, and Richardson LC, et al (2008) Surveillance of cancers associated with tobacco use in the United States from 1999 to 2004 MMWR Surveill Summ 57 1–33 PMID: 18772853
26. Radzikowska E, Głaz P, and Roszkowski K (2002) Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment, and survival: a population-based study of 20 561 cases Ann Oncol 13 1087–1093 https://doi.org/10.1093/annonc/mdf187 PMID: 12176788
27. Bruce N, Dherani M, and Liu R, et al (2015) Does household use of biomass fuel cause lung cancer? A systematic review and evaluation of the evidence for the GBD 2010 study Thorax 70 433–441 https://doi.org/10.1136/thoraxjnl-2014-206625 PMID: 25758120
28. Chen G, Wan X, and Yang G, et al (2015) Traffic-related air pollution and lung cancer: a meta-analysis Thorac Cancer 6 307–318 https://doi.org/10.1111/1759-7714.12185 PMID: 26273377 PMCID: 4448375
29. Lilenbaum RC, Cashy J, and Hensing TA, et al (2008) Prevalence of poor performance status in patients with lung cancer: implications for future research J Thorac Oncol 3 125–129 https://doi.org/10.1097/JTO.0b013e3181622c17 PMID: 18303431
30. Eide IJZ, Nilssen Y, and Stensland EM, et al (2023) Real-world data on EGFR and ALK testing and TKI usage in Norway –a nation-wide population study Cancers 15 1505 https://doi.org/10.3390/cancers15051505
31. Bergethon K, Shaw AT, and Ou SH, et al (2012) ROS1 rearrangements define a unique molecular class of lung cancers J Clin Oncol 30 863–870 https://doi.org/10.1200/JCO.2011.35.6345 PMID: 22215748 PMCID: 3295572
32. Sequist LV, Yang JC, and Yamamoto N, et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations J Clin Oncol 31 3327–3334 https://doi.org/10.1200/JCO.2012.44.2806 PMID: 23816960
33. Kiura K, Imamura F, and Kagamu H, et al (2018) Phase 3 study of ceritinib vs. chemotherapy in patients with ALK-rearranged NSCLC who were previously treated with chemotherapy and crizotinib (ASCEND-5): Japanese subset Jpn J Clin Oncol 48 367–375 https://doi.org/10.1093/jjco/hyy016 PMID: 29474558 PMCID: 5892855
34. Shi Y, Au JS, and Thongprasert S, et al (2014) A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol 9 154–162 https://doi.org/10.1097/JTO.0000000000000033 PMID: 24419411 PMCID: 4132036
35. Noronha V, Prabhash K, and Thavamani A, et al (2013) EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy PLoS One 8 e61561 https://doi.org/10.1371/journal.pone.0061561 PMID: 23620765 PMCID: 3631198
36. Doval DC, Azam S, and Batra U, et al (2013) Epidermal growth factor receptor mutation in lung adenocarcinoma in India: a single-center study J Carcinog 12 12 https://doi.org/10.4103/1477-3163.114970
37. Kota R, Gundeti S, and Gullipalli M, et al (2015) Prevalence and outcome of epidermal growth factor receptor mutations in patients with non-squamous non-small-cell lung cancer Lung India 32 561–565 https://doi.org/10.4103/0970-2113.168099 PMID: 26664160 PMCID: 4663857
38. Pikor LA, Ramnarine VR, and Lam S, et al (2013) Genetic alterations defining NSCLC subtypes and their therapeutic implications Lung Cancer 82 179–189 https://doi.org/10.1016/j.lungcan.2013.07.025 PMID: 24011633
39. Verma S, Kumar M, and Kumari M, et al (2017) An immunohistochemical study of anaplastic lymphoma kinase and epidermal growth factor receptor mutation in non-small cell lung carcinoma J Clin Diagn Res 11 EC22–EC25 PMID: 28892905 PMCID: 5583851
40. Rana V, Ranjan P, and Jagani R, et al (2018) A study of therapy targeted EGFR/ALK mutations in Indian patients with lung adenocarcinoma: a clinical and epidemiological study Med J Armed Forces India 74 148–153 https://doi.org/10.1016/j.mjafi.2017.09.005 PMID: 29692481 PMCID: 5912110
41. Li Y, Appius A, and Pattipaka T, et al (2019) Real-world management of patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer in the USA PLoS One 14(1) e0209709 https://doi.org/10.1371/journal.pone.0209709 PMID: 30608948 PMCID: 6319739
42. Britschgi C, Addeo A, and Rechsteiner M, et al (2020) Real-world treatment patterns and survival outcome in advanced anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer patients Front Oncol 10 1299 https://doi.org/10.3389/fonc.2020.01299 PMID: 32974130 PMCID: 7472246
43. Waterhouse DM, Espirito JL, and Chioda MD, et al (2020) Retrospective observational study of ALK-inhibitor therapy sequencing and outcomes in patients with ALK-positive non-small cell lung cancer Drugs Real World Outcomes 7 261–269 https://doi.org/10.1007/s40801-020-00207-6 PMID: 32725539 PMCID: 7581667
44. Shah M, Noronha V, and Patil V, et al (2024) The role of systemic therapy in patients with advanced non-small cell lung cancer and a poor Eastern Cooperative Oncology Group Performance Status Clin Oncol (R Coll Radiol) 36(2) 128–129 https://doi.org/10.1016/j.clon.2023.12.001






