Evaluation of radiation treatment plan quality in head and neck cancer: a comparative analysis of RapidArc technique with flattening filter and flattening filter-free photon beams
Atul Mishra1,a, Sumanta Manna2,b, Kailash Kumar Mittal1,c, Sharad Singh2,d and Neha Yadav3,e
1Department of Radiation Oncology, Uttar Pradesh University of Medical Sciences, Saifai, Etawah 206130, Uttar Pradesh, India
2Department of Radiation Oncology, Kalyan Singh Super Specialty Cancer Institute, C. G. City, Lucknow 226002, Uttar Pradesh, India
3Department of Radiation Oncology, Apollomedics Super Speciality Hospitals, Lucknow 226012, Uttar Pradesh, India
a https://orcid.org/0000-0002-3077-8628
b https://orcid.org/0000-0003-4079-1591
c https://orcid.org/0000-0001-8770-880X
d https://orcid.org/0009-0007-0607-152X
e https://orcid.org/0000-0002-7712-2415
Abstract
Aim: This study aims to evaluate the treatment plan quality for oral cavity cancers in the head and neck region using the RapidArc (RA) technique with both flattening filter (FF) and flattening filter-free (FFF) photon beams.
Materials and methods: In this analytical study, treatment plans for 12 patients originally planned with a 6 MV FF photon beam were recreated using the RA technique with a 6 MV FFF photon beam. Identical beam parameters and planning objectives were maintained for both sets of plans to facilitate comparison. All plans were evaluated based on planning indices and doses to organs at risk (OAR).
Results: A significant dose variation was found in the minimum (Dmin) and mean (Dmean) doses of the high-risk planning target volume between FF and FFF photon beam RA plans. However, the dose distribution for the low-risk planning target volume was equivalent between the two techniques. The FFF-RA plans demonstrated superior conformity and homogeneity indices compared to the FF plans, with these differences being statistically significant. In addition, the FF-RA plans showed higher doses to the parotid glands, eyes and lenses than the FFF plans. The FFF plans also showed significantly shorter beam-on treatment times and a higher gamma passing index rate compared to the FF plans.
Conclusion: In contrast to the FF photon beam, an FFF photon beam-oriented RA plan provides significant OAR sparing without losing the quality of the treatment plan. High monitor units and beam on time are major highlights of the RA plan with FFF beam.
Keywords: intensity modulated, linear accelerator, radiotherapy, photons, radiotherapy planning, radiotherapy, conformal, FFF, FF, RapidArc
Correspondence to: Atul Mishra
Email: meetatulmishra@gmail.com
Published: 20/02/2025
Received: 25/08/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Head and neck (H&N) cancers have a history of being linked with a late diagnosis and poor prognosis [1]. A significant number of patients have locally aggressive disease that requires multimodality treatment. Oral cavity tumours are a common type of H&N cancer.
Oral cavity tumours are a prevalent type of H&N cancer, accounting for approximately 70% of all reported cases in this category [2]. Radiotherapy remains a crucial component in the treatment of these tumours and plays a vital role in many therapeutic approaches for oral cavity cancer [3].
However, it is incredibly challenging to enhance the local dose for H&N cancer using traditional radiation technology (i.e., parallel-opposed fields with lower anterior neck irradiation for phase 1 and parallel-opposed fields for phase 2); as a result, the 5-year survival rate for H&N cancer remains between 30% and 50%, depending on the stage and location of the tumour [4–7].
The capacity to develop intensity-modulated beams with a multi-leaf collimator and inverse planning enables the use of flattening filter-free (FFF) beams to achieve a more conformal distribution, regardless of beam shape, making the flattening filter (FF) less significant. Furthermore, as Xiao et al [8] pointed out, neither patients nor targets are flat; therefore, FFF fields may be advantageous for moderate or even large targets. The treatment of H&N cancers presents unique challenges because it often affects several critical organs. RapidArc (RA) and intensity-modulated radiation therapy (IMRT) are commonly used treatment methods for these types of cancers because they offer better radiation dose control and help protect nearby critical organs, ultimately leading to improved survival rates and a better quality of life for patients [9]. Numerous studies have explored the practical use of FFF beams, focusing on understanding the properties of FFF beams and assessing their suitability in treatment planning and delivery [10-13]. Radiotherapy machines in contemporary medicine are equipped with FFF beams. Employing FFF beams in plans for treatments such as volumetric modulated arc therapy (VMAT) and IMRT offers numerous potential benefits. These advantages encompass heightened dose delivery rates, diminished collimator scatter, decreased leakage from the machine head and lowered radiation exposure to areas outside the intended treatment field for the patient [14–20]. Cashmore et al [20] documented instances of radiation leakage and reduced peripheral dose when utilising FFF beams in treatment plans targeting the thyroid, lungs, ovaries and testis [21, 22]. However, there have been limited studies conducted on the use of FFF beams for treating H&N cancers, which is insufficient to establish reliable statistical data. Some studies involving larger treatment targets have shown that FFF plans may have less uniformity. In addition, these studies have indicated that FFF beams can yield treatment plan quality and dosimetric parameters similar to FF beams, with no observed improvement in sparing critical organs [21, 22].
To keep up with the evolving sophistication of treatments, quality assurance (QA) for RA has advanced significantly. Various commercial 2D and 3D ionisation chambers or diode detector arrays have recently become popular, enabling quick results to verify absolute dose. Detector arrays are progressively replacing traditional techniques like film dosimetry and point dose measurements in an ionisation chamber. These tools have allowed centres to improve their QA and treat more patients with RA [23]. Moreover, the resolution of detector arrays is restricted [24]. For patient-specific QA, the widely used gamma (γ) passing criteria is a 3 mm distance to agreement (DTA) with a dose difference of 3% and the deviation should be less than 5% in such setting; treatment plans may be delivered without any correction [25, 26].
The current study aims to evaluate the treatment plan quality for oral cavity cancers in the H&N region using the RA technique with both FF and FFF photon beams.
Materials and methods
Twelve patients with H&N cancer who underwent radical resection and required post-operative radiotherapy for oral tongue (anterior tongue) squamous cell carcinomas were selected for this study. Nine males and three females were taken in the current study. The disease was staged as per the American Joint Committee on Cancer Staging Guidelines 8th edition (2017), and stagewise distribution was: Stage II - Three patients, Stage III - Seven patients and Stage IV - Two patients. All twelve patients were simulated with a computed tomography (CT) simulator in the supine position. The CT simulations were performed with a helical scanner with a 3.0 mm slice thickness. The tumour volumes, gross tumour volume, clinical target volume (CTV), planning target volume (PTV) and contouring of organs at risk (OARs) were done according to RTOG protocol [27]. Two sets of RA with dual-arc plans were performed with jaw tracking using 6-MV FF and FFF beams. All RA plans were generated using the Eclipse treatment planning system (v15.6; Varian Medical Systems, Palo Alto, CA, USA). A photon optimiser (PO; Version15.6.06, Varian Medical Systems) was selected for inverse optimisation based on physical and biological objectives with a 2.5 mm dose grid resolution and Anisotropic Analytical Algorithm (AAA). All plans were generated for PTV high risk (PTV_HR) 60Gy/30# and PTV low risk (PTV_LR) 54Gy/30#, a uniform 5 mm margin was taken around the CTV to create the PTV in all cases. Dose constraints given for OAR planning were Parotid Gland (Both) mean dose <25 Gy, Brainstem Dmax <54 Gy, Eyes (globe) Mean <35 Gy and Dmax <54 Gy and Lens Dmax <7 Gy. The spinal cord (SC) dose limit (Dmax) was kept at <45 Gy. A Varian TrueBeam accelerator equipped with 120 leaves Millennium multi-leaf collimator (M120, MLC) was used to develop all RA plans with a maximum dose rate of 600 monitor units (MU)/min and 1,400 MU/min for the FF and FFF photon beams, respectively. Patient plan optimisation and sequencing parameters were kept as per standard protocol.
Plan assessment and validation
All RadpidArc plans (with FF and FFF) were evaluated for HI, CI, Dmax, Dmean, D98, D50, D2, V95, V107 and dose to OAR using dose volume histogram (DVH). Gradient index (GI) is an indicator of dose fall-off. It assesses the dose of radiation gradient beyond the target. GI was defined as a ratio of V95% prescription isodose dose (PID) over V50% PID [28, 29]. Low GI and High Gradient Index were defined as the ratio of V25% PID over V50% PID and V50% PID over V90% PID, respectively, where V25%, V50% andV90% were volumes receiving 25%, 50% and 90% of the PID, respectively [28, 29].
The doses to the OARs were recorded for all 12 patients, with mean ± standard deviation (SD) values for each patient's plans. The doses calculated by the treatment planning system (TPS) were compared with measured doses using patient-specific QA. In this study, patient-specific QA for RA used a 3 mm DTA and 3% dose difference criteria, with a threshold value set at 5% [25]. Therefore, both FF and FFF RA plans were irradiated and compared using an electronic portal imaging detector (EPID). Furthermore, the dose difference analysis was defined as the TPS-calculated dose at a specific point minus the measured dose at the same point, divided by the measured dose at that point (Equation 1) [26].
PV= {[TPSPD (FF or FFF) −Measured Dose (EPID)]/ Measured Dose (EPID)} ×100 (1)
Where PV defines percentage variation; TPSPD, TPS Planned Dose; FF, flattened filter; FFF, FFF and EPID, Electronic Portal Imaging Detector for equation 1.
Statistical analysis
The mean and SD were calculated for continuous variables to summarise the central tendency and dispersion of the data. Comparisons between the Rapid with FF and RA with FFF groups were performed using two-tailed paired t-tests. Statistical analyses were conducted using R programming software. A p-value of less than 0.05 was considered statistically significant.
Results
In the current study, PTV_HR and PTV_LR mean volumes were 265.12 ± 64.65 and 385.41 ± 112.84 cc, respectively. The average PTV coverage for all patients in both FF and FFF RA techniques could achieve 95% of the prescription dose (60 and 54 Gy) to 95% of the PTV and didn’t exceed 109%. Figure 1 shows the dose colour wash distribution for FF and FFF beam plans.
Figure 1 displays the dose colour wash distribution covering the high-risk (cyan) and low-risk PTV (orange). In the figure, A–C represent plan distributions with FF beams in the three planes (axial, coronal and sagittal), while D–F represent plan distributions with FFF beams.
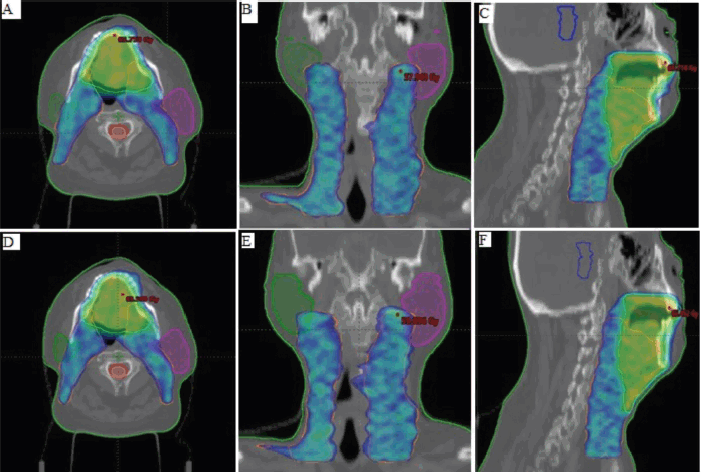
Figure 1. Displays the dose colourwash distribution covering the high-risk (cyan) and low-risk PTV (orange). In the figure, A–C represent plan distributions with FF beams in the three planes (axial, coronal and sagittal), while D–F represent plan distributions with FFF beams.
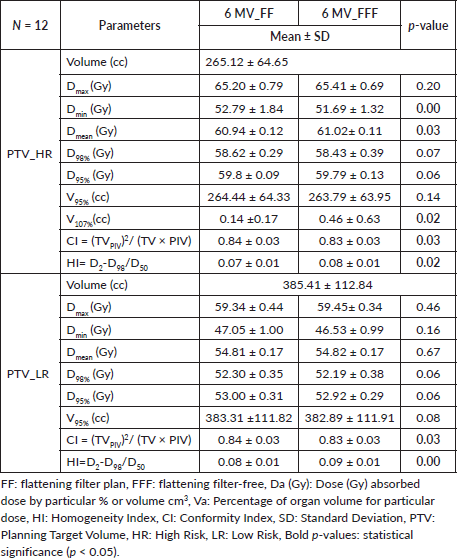
The D98, D95, D2, D50, Dmean, Dmax, Dmin, V95 and V107 were recorded for PTV_HR and PTV_LR (Table 1). All these values were found statistically not significant except Dmin, V107 and Dmean for PTV_HR of FF and FFF plans. The average HI values were found to be 0.07 ± 0.01 and 0.08 ± 0.01 for FF and FFF of PTV_HR, respectively. The average HI values were found to be 0.08 ± 0.01 and 0.09 ± 0.01 for FF and FFF of PTV_LR, respectively. HI values for both PTVs were found statistically significant (p = 0.02 for PTV_HR and p = 0.00 for PTV_LR). The average CI values were found similar for both PTVs for FF and FFF, respectively, which is statistically significant (p = 0.03). Table 2 presents the dosimetric parameters for normal tissue and OARs for all patients.
Statistically significant differences were observed in normal tissue parameters such as V90, V50 and low dose gradient index (LGI). However, parameters such as V95, V25, GI and high dose gradient index (HGI) showed no statistically significant differences between both techniques. Figure 2 illustrates the DVHs for PTVs and OARs of FF and FFF beam plans.
The mean dose to the right parotid gland was significantly higher with FF RA plans (26.14 ± 1.08 Gy) compared to FFF RA plans (26.02 ± 1.02 Gy) (p = 0.05). Similarly, the mean dose to the left parotid gland was significantly higher with FF RA plans (26.04 ± 2.65 Gy) compared to FFF RA plans (25.84 ± 2.60 Gy) (p = 0.02). FFF plans showed a significant reduction in the right eye and left eye dose than FF plans and were found to be statistically significant (p = 0.04 for the right eye and p = 0.05 for left eye). Eye lenses (both right and left) were shown less for FFF techniques and statistically significant (p = 0.04). SC max dose was within the tolerance limit for FF and FFF techniques (p = 0.83). Statistical variations of OAR dose are represented in Figures 3 and 4.
Table 1. Dosimetric and plan quality indices for the PTV using 6 MV FF and 6 MV FFF RA plans.
Brainstem max dose was reported for all 12 patients and was 30.82 ± 6.17 and 30.79 ± 6.18 Gy for FF and FFF RA, respectively, and statistically not significant (p = 0.78).
Gamma passing criterion
All the plans for the 12 patients were verified using EPID (a-Si 1200 portal imager).
The results showed that the gamma passing rate (the passing criteria were 3% dose difference at 3 mm DTA) of the SD in the different plans was 97% or higher.
The gamma passing rate for FFF increased by 1.76% compared to FF, i.e., 99.28% versus 97.56%, respectively. Figure 5 represents the gamma index passing values for different patients.
Table 2. Dosimetric parameters for normal tissue and OARs.
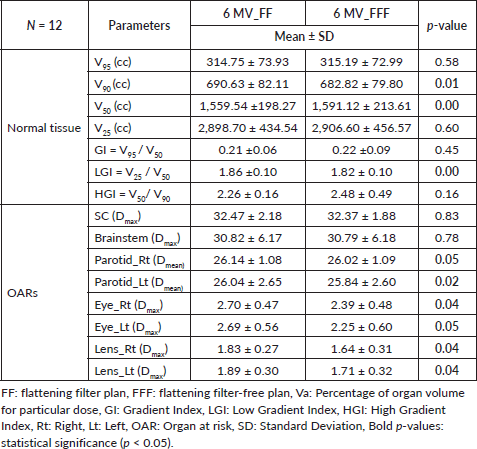
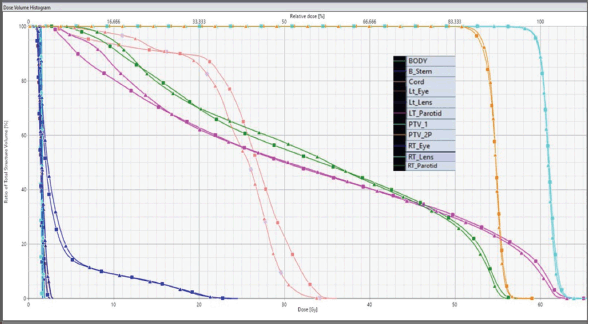
Figure 2. DVHs for PTVs and OARs. Lines with triangles represent FF data, and those with squares represent FFF data.
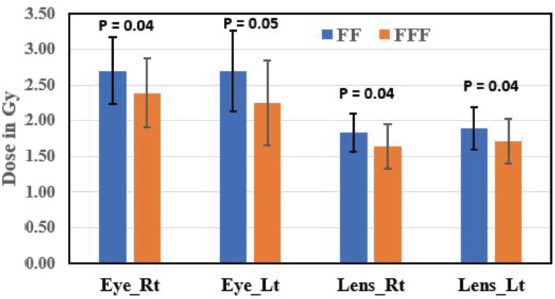
Figure 3. A bar chart comparing the dose (Mean ± SD) in Gy to the OAR, for the right eye (Eye_Rt), left eye (Eye_Lt), right lens (Lens_Rt) and left lens (Lens_Lt), in both FF and FFF plans.
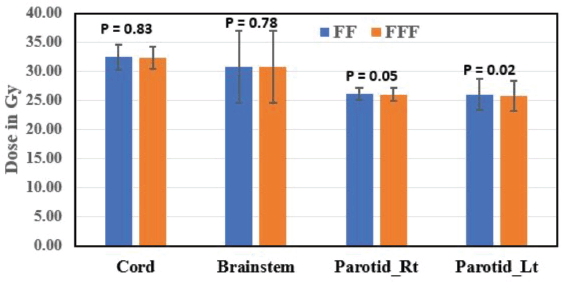
Figure 4. A bar chart comparing the dose (Mean ± SD) in Gy to the OAR, cord, brainstem, parotid right (Parotid_Rt) and parotid left (Parotid_Lt), in both FF and FFF plans.
MU and treatment time (TT)
For the two techniques in this study, the variations in cumulative MUs were statistically significant (p = 0.00). FFF RA (697.18 MU) was increased by 6.41% relative to FF RA (655.22 MU) on the mean for a variation.
The MU of the FF RA plan in the H&N region was noticeably lower compared to the FFF RA plan, as shown in Table 3 (p = 0.00).
The difference between the two plans' beam on time (BOT) (minutes) was statically significant, with the FFF plan (0.90 minutes) being, on average, 49.15% faster than the FF plan (1.77 minutes) (p = 0.00; Table 3). The total beam-on time of FF and FFF for different patients as shown in Figure 6.
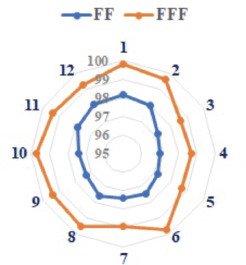
Figure 5. Variation of percentage of gamma passing rate for FF and FFF beam with AAA dose.
Table 3. Comparison of MU, BOT and gamma passing rate (GP) for 6 MV FF and 6 MV FFF RA plans.
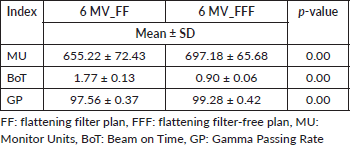
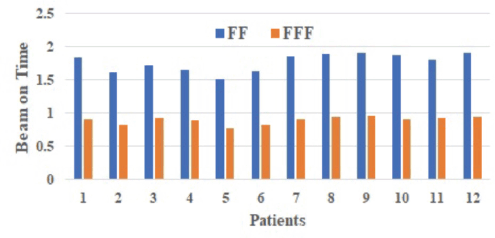
Figure 6. Total BOT of FF and FFF for different patients computation algorithm.
Discussion
The current study evaluates the radiation treatment plan quality of the H&N for FF and FFF radiotherapy plans based on different dosimetric parameters and plan validation with patient-specific QA using EPID.
Presently, the FFF RA technique has been used to treat the H&N, the lungs and a number of other regions. When compared to other radiation therapy approaches, the FFF RA technique may help reduce TT while also achieving better radiobiological impacts [21, 22]. Furthermore, there has been very little evidence of such a study being employed on patients with H&N cancer. It remains a significant challenge to improve target prescribed dose coverage, dosage homogeneity and conformity and reduce dose to OARs in H&N malignancies.
In the present study, authors found that FF and FFF RA techniques were able to achieve greater than 95% of the prescription dose (60 and 54 Gy) to 95% of the PTV and less than 109%. Kumar et al [2] reported similar outcomes utilising the FF and FFF RA techniques for H&N patients. Saroj et al [30] reported HI for FFF plans was better than FF plans and CI was clinically insignificant. In the current study, FFF plans show better HI, which is statistically significant (p = 0.02), and FF RA plans show better CI than FFF RA plans, which is statistically significant (p = 0.03). Manna et al [31] conducted a study on brain neoplasms planned with 6 MV FFF and VMAT and found superior conformity of the dose to the PTV, as well as a reduction in the dose to the eyes and optic nerve. In addition, there was a significant reduction in low-dose volumes and integral doses. In a similar study, Manna et al [32] demonstrated that for cervical cancer treatment, the use of the FFF beam and RA technique resulted in a statistically significant improvement in the conformity index, with an observed difference of 3.06%. FFF beams are clinically advantageous in the treatment of lung cancer as well as in radiation therapy for synchronous bilateral breast carcinoma [33, 34].
Kumar et al [29] reported that parotid, eye and lens doses were similar and statistically insignificant for H&N RA FF and FFF plans. However, in the present study shows a lesser dose in parotid (Parotid_Rt: p = 0.05, Parotid_Lt: p = 0.02), eye (Eye_Rt: p = 0.04, Eye_Lt: p = 0.05) and lens (Lens_Rt: p = 0.04, Lens_Lt: p = 0.04) for FFF RA plans and statistically significant. Kumar et al [29] reported that GI for FF and FFF plans were 3.85 and 3.87, respectively, and statistically insignificant (p = 0.96). In the present study, a similar trend was observed for GI and less LGI for FFF over FF plans, statistically significant (p = 0.96). However, HGI was statistically insignificant for FF and FFF RA plans (p = 0.16).
Saroj et al [35] compared the quality of IMRT treatment plans for cancer of the esophageal with and without FF photon beams. The IMRT plan with FFF beam offers significant benefits, including high MUs and less BOT. In this investigation, the MUs exhibited a statistically significant difference (p = 0.00). The quantity of MUs for the RA FF plan (655.22 ± 72.43 MU) was found to be lesser compared to the RA FFF plan (697.18 ± 65.68 MU). The BOT between the two plans was also statistically significant. Specifically, RA FFF plans demonstrated a shorter BOT (0.90 ± 0.06 minutes) compared to the longer BOT (1.77 ± 0.13 minutes) for RA FF plans (p = 0.00). The observed increase in MUs for FFF-RA plans, as opposed to FF-RA plans, might be attributed to the necessity for increased modulation when utilising FFF beams to ensure uniform dose distribution in larger tumours, resulting in a higher MU count. Nevertheless, the higher dose rate associated with FFF beams contributes to an overall reduction in beam delivery time. Reducing treatment delivery time would be beneficial for patients susceptible to motion.
As per recommendations, SC and brainstem dose (45 and 54 Gy, respectively) were both efficacious and met the specified tolerance criteria [29, 36]. In the current study, the authors stated that the mean dose to the two essential organs (SC and Brainstem) for RA FFF plans accomplished the above criteria and slightly decreased dose as compared to RA FF plans (p = 0.83 and p = 0.78, respectively).
AAPM task group 119 suggests an appropriate threshold indicated as a percentage of points meeting gamma criteria of 3%/3 mm: 94.2% for individual field assessments and 92.4% for combined irradiations assessed using radiographic film [25, 37–39]. Ji et al [40] evaluated the feasibility of using FFF beams for whole-brain radiotherapy while sparing the hippocampus. They assessed target and organ-at-risk parameters for both FF and FFF beams and concluded that the differences in the gamma index were negligible. In the current study, we found that the mean gamma index passing criteria was greater than 97% at 3%/3 mm for both techniques, which shows good results of our plans. FFF was superior to FF with a betterment of 1.76% (99.28% versus 97.56%).
The limitations of the study included a small number of participants and an inequitable distribution of male as well as female patients. It is therefore recommended that a newer study be conducted with a larger number of participants for high validity and that long-term clinical follow-up may also be required to determine whatever radiotherapy toxicities. Subsequent studies could explore potential radiobiological effects and further investigate the FFF technique to minimise TT. The rapid advancement in the radiation treatment of mid and advanced H&N cancers suggests promising avenues for future research.
Conclusion
From the present study, it can be concluded that FFF-based RA plans were better than FF plans in the context of target coverage, OAR constraints, HI and CI. Moreover, FFF RA plans to prove to be effective in reducing intrafraction errors, particularly in H&N tumour cases, as they exhibit significantly shorter TT compared to FF plans.
Conflicts of interest
The authors state that they have no conflicting interests in this manuscript.
Funding
No funding was received for this work.
Ethical approval
Since this is a retrospective study involving previously treated patients, ethical approval was exempted by the institute.
References
1. Kumar M, Nanavati R, and Modi TG, et al (2016) Oral cancer: etiology and risk factors: a review J Can Res Ther 12 458–631 https://doi.org/10.4103/0973-1482.186696
2. Kumar SA, Musthafa MM, and Suja CA, et al (2021) Dosimetric comparison of FF and FFF beams in VMAT treatment plans of head and neck cancers Onkologia i Radioterapia 15(7) 1–5
3. Dai X, Zhao Y, and Liang Z, et al (2015) Volumetric modulated arc therapy for oropharyngeal carcinoma: a dosimetric and delivery efficiency comparison with static-field IMRT Phys Med 31 54–59 https://doi.org/10.1016/j.ejmp.2014.09.003
4. Hoesseini A, Offerman MPJ, and van de Wall-Neecke BJ, et al (2020) Physicians’ clinical prediction of survival in head and neck cancer patients in the palliative phase BMC Palliat Care 19 176 https://doi.org/10.1186/s12904-020-00682-2
5. Parvathaneni U, Laramore GE, and Liao JJ (2012) Technical advances and pitfalls in head and neck radiotherapy J Oncol 2012 597467 [doi: 10.1155/2012/597467] https://doi.org/10.1155/2012/597467 PMID: 22701482 PMCID: 3369487
6. Abdel-Hakim K, Nishimura T, and Takai M, et al (2005) Review of monoisocentric split-field technique for conventional and IMRT treatment in head and neck cancers: technical limitations and approaches for optimization Technol Cancer Res Treat 4(1) 107–113 https://doi.org/10.1177/153303460500400114 PMID: 15649094
7. Kachhwaha A, Tiwari R, and Gayen S, et al (2023) Comparison of sequential versus simultaneous integrated boost of volumetric modulated arc therapy in treatment of oropharyngeal carcinoma Cancer Treat Res Commun 36 100721 https://doi.org/10.1016/j.ctarc.2023.100721 PMID: 37301126
8. Xiao Y, Kry SF, and Popple R, et al (2015) Flattening filterfree accelerators: a report from the AAPM Therapy Emerging Technology Assessment Work Group J Appl Clin Med Phys 16 5219 https://doi.org/10.1120/jacmp.v16i3.5219
9. Infusino E (2015) Clinical utility of RapidArc™ radiotherapy technology Cancer Manag Res 7 345–356 https://doi.org/10.2147/CMAR.S72775 PMCID: 4648597
10. Syam Kumar SA, Vivekanandan N, and Sriram P (2012) A study on conventional IMRT and RapidArc treatment planning techniques for head and neck cancers Rep Pract Oncol Radiother 17(3) 168–175 https://doi.org/10.1016/j.rpor.2012.01.009 PMID: 24377020 PMCID: 3863199
11. Pönisch F, Titt U, and Vassiliev ON, et al (2066) Properties of unflattened photon beams shaped by a multileaf collimator Med Phys 33 1738–1746 https://doi.org/10.1118/1.2201149
12. Scorsetti M, Alongi F, and Castiglioni S, et al (2011) Feasibility and early clinical assessment of flattening filter free (FFF) based stereotactic body radiotherapy (SBRT) treatments Radiat Oncol 6 113 https://doi.org/10.1186/1748-717X-6-113 PMID: 21910868 PMCID: 3179946
13. Georg D, Knoos T, and McClean B (2011) Current status and future perspective of flattening filter free photon beams Med Phys 38 1280–1293 https://doi.org/10.1118/1.3554643 PMID: 21520840
14. Mani KR, Bhuiyan MA, and Rahman MS, et al (2018) Open beam dosimetric characteristics of true beam medical linear accelerator with flattening filter (WFF) and flattening filter free (FFF) beam Pol J Med Phys Eng 24 79–89 https://doi.org/10.2478/pjmpe-2018-0011
15. Nicolini G, Ghosh-Laskar S, and Shrivastava SK, et al (2012) Volumetric modulation arc radiotherapy with flattening filter-free beams compared with static gantry IMRT and 3D conformal radiotherapy for advanced esophageal cancer: a feasibility study Intern J Radiat Oncol Biol Phys 84 553–560 https://doi.org/10.1016/j.ijrobp.2011.12.041
16. Huang Y, Siochi RA, and Bayouth JE (2012) Dosimetric properties of a beam quality-matched 6 MV unflattened photon beam J Appl Clin Med Phys 13 3701 https://doi.org/10.1120/jacmp.v13i4.3701 PMID: 22766941 PMCID: 5716519
17. Subramanian S, Thirumalaiswamy S, and Srinivas C, et al (2012) Chest wall radiotherapy with volumetric modulated arcs and the potential role of flattening filter free photon beams Strahlenther Onkol 188 484–490 https://doi.org/10.1007/s00066-012-0075-6
18. Yan Y, Yadav P, and Bassetti M, et al (2016) Dosimetric differences in flattened and flattening filter-free beam treatment plans J Med Phys 41 92–99 https://doi.org/10.4103/0971-6203.181636 PMID: 27217620 PMCID: 4871009
19. Sun WZ, Chen L, and Yang X, et al (2018) Comparison of treatment plan quality of VMAT for esophageal carcinoma with flattening filter beam versus flattening filter-free beam J Cancer 9 3263–3268 https://doi.org/10.7150/jca.26044 PMCID: 6160692
20. Cashmore J, Ramtohul M, and Ford D (2011) Lowering whole-body radiation doses in pediatric intensity-modulated radiotherapy through the use of unflattened photon beams Int J Radiat Oncol Biol Phys 80 1220–1227 https://doi.org/10.1016/j.ijrobp.2010.10.002
21. Gasic D, Ohlhues L, and Brodin NP, et al (2014) A treatment planning and delivery comparison of volumetric modulated arc therapy with or without flattening filter for gliomas, brain metastases, prostate, head/neck, and early-stage lung cancer Acta Oncol 53 1005–1011 https://doi.org/10.3109/0284186X.2014.925578 PMID: 24937551
22. Zhuang M, Zhang T, and Chen Z, et al (2013) Advanced nasopharyngeal carcinoma radiotherapy with volumetric modulated arcs and the potential role of flattening filter-free beams Radiat Oncol 8 120 https://doi.org/10.1186/1748-717X-8-120 PMID: 23672519 PMCID: 3720531
23. Mishra A, Pathak R, and Mittal KK, et al (2024) Efficacy of the collapsed cone algorithm calculated radiotherapy plans in intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT): a comparative dosimetric study in tumors of the thorax J Cancer Res Ther 20(1) 383–388 https://doi.org/10.4103/jcrt.jcrt_2171_22 PMID: 38554350
24. Hussein M, Adams EJ, and Jordan TJ, et al (2013) A critical evaluation of the PTW 2D-ARRAY seven29 and OCTAVIUS II phantom for IMRT and VMAT verification J Appl Clin Med Phys 14(6) 4460 https://doi.org/10.1120/jacmp.v14i6.4460 PMID: 24257288 PMCID: 5714639
25. Miften M, Olch A, and Mihailidis D, et al (2018) Tolerance limits and methodologies for IMRT measurement-based verification QA: recommendations of AAPM Task Group No. 218 Med Phys 45 e53–e83 https://doi.org/10.1002/mp.12810
26. Mishra A, Pathak R, and Raj Verma T, et al (2023) Evaluation of radiation treatment planning algorithms in IMRT and VMAT: a comparative dosimetric study in lung equivalent heterogeneous medium J Biomed Phys Eng 13(6) 503–514 PMID: 38148960 PMCID: 10749411
27. Grégoire V, Ang K, and Budach W, et al (2014) Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines Radiot Oncol 110(1) 172–181 https://doi.org/10.1016/j.radonc.2013.10.010
28. Paddick I and Lippitz B (2006) A simple dose gradient measurement tool to complement the conformity index J Neurosurg 105(Suppl) 194–201 https://doi.org/10.3171/sup.2006.105.7.194 PMID: 18503356
29. Kumar A, Sharma K, and Bhatt CP, et al (2023) Dosimetric comparison of unmatched flattening filter-free and flattened beams in volumetric arc therapy plans for head-and-neck cancer J Med Phys 48(4) 338–344 https://doi.org/10.4103/jmp.jmp_68_23
30. Saroj D, Yadav S, and Paliwal N, et al (2024) Radiobiological analysis of VMAT treatment plan with flattened and flattening filter free photon beam: an EUD and TCP based comparative study Rep Pract Oncol Radiother 29(1) 77–89 PMCID: 11333070
31. Manna S, Kombathula SH, and Gayen S, et al (2021) Dosimetric impact of FFF over FF beam using VMAT for brain neoplasms treated with radiotherapy Polish J Med Phys Eng 27(3) 191–199 https://doi.org/10.2478/pjmpe-2021-0023
32. Manna S, Singh S, and Gupta PK, et al (2023) Dosimetric and radiobiological impact of flattening filter-free beam and dose calculation algorithm using RapidArc plans for cervical cancer treatment Prec Radiat Oncol 7 197–206 https://doi.org/10.1002/pro6.1207
33. Alfishawy MM, Kany AI, and Elshahat KM (2024) Impact of flattening filter-free beams on remaining volume at risk in lung cancer treatment Radiat Environ Biophys 63(3) 455–464 https://doi.org/10.1007/s00411-024-01073-4 PMID: 38762614
34. Zhang X, Shi J, and Wu X, et al (2024) Dosimetric comparison of commonly used volumetric modulated arc therapy field arrangements based on flattening filter-free beams for synchronous bilateral breast carcinoma radiation therapy Pract Radiat Oncol 14(3) e190–e202 https://doi.org/10.1016/j.prro.2023.11.002
35. Saroj DK, Yadav S, and Paliwal N, et al (2023) Assessment of treatment plan quality between flattening filter and flattening filter free photon beam for carcinoma of the esophagus with IMRT technique J Biomed Phys Eng 13 227–238 PMID: 37312893 PMCID: 10258210
36. Wu AJ, Bosch WR, and Chang DT, et al (2015) Expert consensus contouring guidelines for intensity modulated radiation therapy in esophageal and gastroesophageal junction cancer Int J Radiat Oncol Biol Phys 92(4) 911–920 https://doi.org/10.1016/j.ijrobp.2015.03.030 PMID: 26104943 PMCID: 4481325
37. Ezzell GA, Burmeister JW, and Dogan N, et al (2009) IMRT commissioning: multiple institution planning and dosimetry comparisons, a report from AAPM task group 119 Med Phys 36(11) 5359–5373 https://doi.org/10.1118/1.3238104 PMID: 19994544
38. Verma T, Painuly NK, and Mishra SP, et al (2016) Performance evaluation of algorithms in lung IMRT: a comparison of Monte Carlo, pencil beam, superposition, fast superposition and convolution algorithms J Biomed Phys Eng 6(3) 127–138 PMCID: 5106545
39. Das S, Kharade V, and Pandey VP, et al (2022) Gamma index analysis as a patient-specific quality assurance tool for high-precision radiotherapy: a clinical perspective of single institute experience Cureus 14(10) e30885 PMID: 36337776 PMCID: 9626372
40. Ji T, Sun L, and Cai F, et al (2022) Comparison between flattening filter‑free (FFF) and flattened photon beam VMAT plans for the whole brain radiotherapy (WBRT) with hippocampus sparing Asia Pac J Clin Oncol 18 e263–e267 https://doi.org/10.1111/ajco.13624





