The study of elastic fibres in oral precancerous and cancerous lesions using Shikata’s modified orcein stain: a retrospective study
Sandhya Tamgadge1, Nikita Kamble1, Treville Pereira1, Avinash Tamgadge1, Mayura Chande1 and Siddharth Acharya2
1Department of Oral and Maxillofacial Pathology and Microbiology, DY Patil University School of Dentistry, Sector 7, Nerul, Navi Mumbai 400706, Maharashtra, India
2Department of Public Health Dentistry, DY Patil University School of Dentistry, Nerul Navi Mumbai, Sector 7, Nerul, Navi Mumbai 400706, Maharashtra, India
Abstract
Statement of the problem: Oral squamous cell carcinoma (OSCC) is the most common type of oral cancer. During the invasion, tumour cells break through the basement membrane and penetrate the connective tissue to interact with the extracellular matrix. An attempt was made to evaluate the connective tissue changes in different grades of OSCCs, oral submucous fibrosis (OSMF) and Oral Epithelial Dysplasias. Literature related to the evaluation of malignant epithelial cells is vast but very sparse knowledge is available on the role of extracellular stromal fibres on tumour invasion in oral cancer and precancer especially on elastic fibres using Shikata’s Modified Orcein Stain.
Purpose: To analyse the changes in elastic fibres in varying grades of OSCC, OSMF and Oral Epithelial Dysplasias using a special stain, Shikata’s modified orcein stain.
Materials and method: A total of 100 cases were selected as the study group in this retrospective observational study. One section each was cut from 50 samples of varying grades of OSCC and 50 samples of varying grades of OSMF and oral leukoplakia. Ten samples of the control group were taken from the archives of the Department of Oral Pathology. Qualitative and quantitative analysis of elastic fibres was accomplished using set criteria. Spearman’s test was done to evaluate the elastic fibres. Statistically insignificant results were obtained for quantitative and qualitative analysis of elastic fibres.
Results: A change in density in elastic fibres was observed in progression from early to advanced grades of OSCC, OSMF and oral epithelial dysplasia cases. A low expression of elastic fibres in tumour stoma indicates that the protective barrier against tumour progression is deficient, allowing the tumour to progress more easily and resulting in a poorer prognosis.
Conclusion: The elastic fibres undergo a change in density, orientation and packing in the stroma of varying grades of OSCC, OSMF and oral epithelial dysplasia cases. The unique feature of this study lies in the exploration of elastic fibres in OSCC which has not been done so far.
Keywords: elastic fibres, OSCC, OSMF, oral epithelial dysplasias, Shikata’s modified orcein stain, precancer, cancer
Correspondence to:Sandhya Tamgadge
Email: sandhya.tamgadge@gmail.com
Published: 14/11/2024
Received: 18/05/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Oral cavity cancer is a major global health concern. Squamous cell carcinoma is a malignant epithelial cell neoplasm exhibiting squamous differentiation as characterised by the formation of keratin and/or the presence of intercellular bridges [1–3]. When a potentially malignant disorder progresses to cancer it undergoes series of histological alterations [4, 5]. The stroma especially stromal fibres plays an important role in the invasion and metastasis [6]. The role of mesenchyme in tumours can indicate the ability of the tumour cells to invade and metastasize [7]. Morphologic detection on haematoxylin and eosin and special stains of connective tissue fibres are cost-effective compared to the molecular markers. Invasion and metastasis can be studied effectively by the surgeon with the use of special stains. There are multiple studies on collagen and its association with metastasis but the association of tumour cells and elastic fibres and its effect on the progression of carcinoma is questionable [8]. Tuxhorn et al [9] quoted that the cancer cells can interact specifically with elastin through two elastin-binding proteins and galectin-3. Timar et al [10] Sabnis et al [11] and Kielty et al [12] have suggested that there is a positive correlation between tumour progression and the presence of elastic fibres in the tumour stroma [10–12].
The advantages of the use of orcein for staining elastic fibres in the skin were described in 1891 by Unna [13]. Henwood mentioned that various unpublished studies on elastic tissues failed to demonstrate elastic fibres including the Verhoeffs Van Giesson stain. Therefore, Henwood used Shikata’s orcein technique in his research to demonstrate the hepatitis B surface antigen (HBsAg), copper-associated protein (CAP) and sulfated mucins, which also stained the elastic fibres in the areas of liver fibrosis [14]. In dental literature, Shikata’s modified orcein stain has been used only in lichen sclerosis of the lip [15].
Shikata’s modified orcein stain has been used to study the elastic tissue by Henwood [16, 17].
Verhoeff–van Giesson’s Elastic Stain is used to identify the atrophy of elastic tissue, such as in emphysema, evidence of vascular diseases (arteriosclerosis) and the invasion of tumours into vessels.
In the literature only 2 studies, one by Dineshshankar et al [18] and another by John and Murthy [19] have been done to study the morphology of elastic fibres using special stains. They are as follows: Dineshshankar et al [18] conducted a comprehensive investigation to evaluate the morphological alterations in elastic fibres, a principal component of connective tissue, across varying grades of oral squamous cell carcinoma (OSCC) and epithelial Oral Epithelial Dysplasias. Their study provided valuable insights into the progressive changes in elastic fibre architecture during oral carcinogenesis [18].
In a related study, John and Murthy [19] examined both collagen and elastic fibres at different stages of OSCC. Their research aimed to correlate these stromal changes with two well-established grading systems: Broder’s and Bryne’s. This comparative approach offered a nuanced understanding of how extracellular matrix (ECM) alterations relate to traditional histopathological grading methods in OSCC [19].
Notably, Katsoulas et al [15] employed Shikata’s modified orcein stain in their study of lichen sclerosus of the lip. Their use of this staining technique demonstrates the versatility and potential broader applications of Shikata’s modified orcein stain in visualising elastic fibre changes across various oral lesions [15].
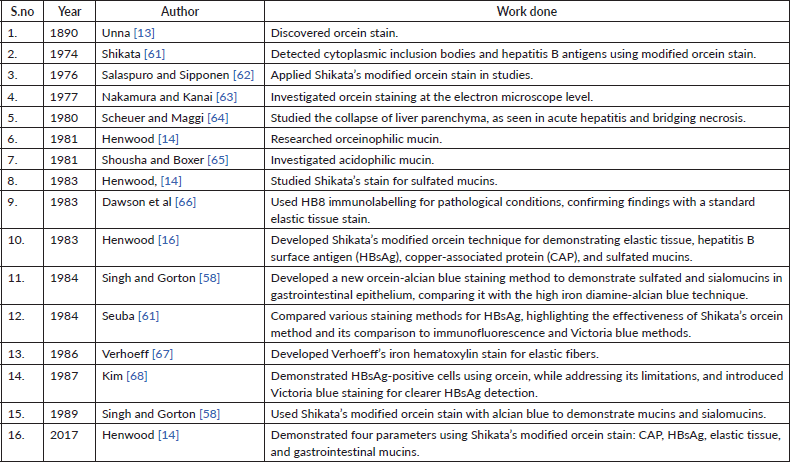
These studies collectively underscore the growing interest and importance of investigating elastic fibre alterations in oral pathologies. They highlight the potential of elastic fibre assessment as a valuable tool in understanding disease progression and potentially aiding in the diagnosis and prognosis of oral lesions. The sparse literature, the lesser number of elastic fibres in lamina propria and the masking effect of overlying inflammatory cells could be the limiting factors in the assessment of elastic fibres in OSCC. This calls for more sensitive labelling systems for assessing elastic fibres (Tables 1 and 2).
This could be is the first study in the literature in which elastic fibres have been evaluated in precancer and cancer using Shikata’s modified orcein stain.
Aims and objectives
To study the elastic fibres in oral potentially malignant disorders and oral cancerous lesions using Shikata’s modified orcein stain and to correlate with the histological grading using hematoxylin and eosin.
Table 1. Shows timeline of various elastic stains.
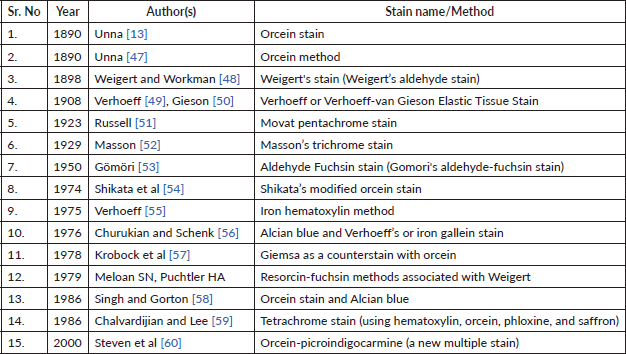
Table 2. Various combinations of orcein stain and other stains used for different tissue components.
Materials and method
Ethical approval was obtained from the Institutional Ethics Committee before the commencement of the study. The IREB Reference Number is IREB/2020/OP/01.
The study was conducted over 1 year, from January to December 2021. Histological sections were obtained from the archives of the Department of Oral Pathology and Microbiology. As controls, ten cases of normal oral mucosa were selected, along with a transverse section of the femoral artery as a positive control.
The study group comprised diagnosed cases of OSCC and potentially malignant disorders, including oral submucous fibrosis (OSMF) and oral leukoplakia (oral epithelial dysplasia). The inclusion criteria were histologically confirmed cases with sufficient epithelial and connective tissue. Cases were divided into two categories OSCC: cancerous and precancerous OSCC. The cancerous group OSCC included 50 cases of OSCC, which were further classified into 30 well-differentiated OSCC, 15 moderately differentiated and 5 poorly differentiated cases according to Broder’s classification [20]. The precancerous group included 21 cases of leukoplakia categorised by the severity of epithelial dysplasia (9 mild, 7 moderate and 5 severe) and 29 cases of OSMF were graded as follows: 18 cases in Grade I, 6 in Grade II, 3 in Grade III and 2 in Grade IV, based on the system proposed by Rajendran et al [21].
Paraffin-embedded tissue sections were retrieved from the archives for further analysis. A single section was prepared from each paraffin block and stained using Shikata’s modified orcein stain, following the technique described by Henwood [14] to assess the elastic fibre component. The elastic fibres were analysed at magnifications of 4×, 10× and 40× for their density, grouping patterns, staining intensity and orientation relative to the overlying epithelium and tumour islands using a Leica research microscope (Model No. DM1000 LED, Leica Microsystems GmbH, Ernst-Leitz-Straße 17–37, 35578 Wetzlar, Germany). Photomicrographs were captured for documentation purposes.
The density of elastic fibres was categorised as scanty, moderate or abundant. Morphological patterns were classified as long, thin, straight fibres present singly or short, thick, wavy fibres present in clusters. The staining intensity, the orientation of elastic fibres to the overlying epithelium and their orientation to tumour islands were carefully evaluated [22].
All histological slides were reviewed independently by two pathologists who were blinded to the patients’ clinical details. In cases of discrepancy, a consensus was reached using a multi-headed microscope. A minimum of four fields of view at 200× magnification were selected for analysis, and digital images were captured for further evaluation. Statistical analysis of elastic fibre parameters across various grades of OSCC, OSMF and oral leukoplakia was performed using Spearman’s test.
Results
Under microscopic examination, the elastic fibres were observed as delicate, undulating structures. These fibres were predominantly located within the connective tissue matrix, forming an intricate network throughout the examined tissue sections. Their distinctive wavy appearance and distribution pattern were characteristic features that aided in their identification and analysis.
Colour of elastic fibres
The elastic fibres stained a deep brown to dark purple colour in the tissue sections, providing a distinct contrast against the background tissue, which stained a lighter shade. This coloration facilitated the clear identification and assessment of the elastic fibres within the oral precancerous and cancerous lesions and positive control sample that is artery wall.
Various morphologic patterns of elastic fibres were observed under the Leica microscope (Tables 3–6).
Table 3. Density of elastic fibre in cancer versus precancer.
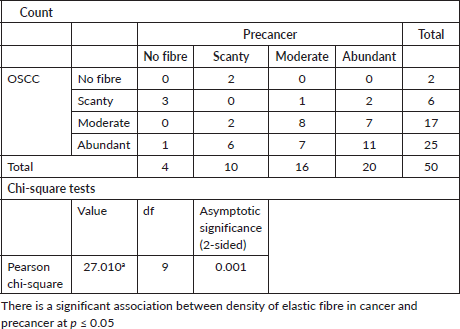
Table 4. Pattern of grouping of elastic fibres in cancer versus in precancer.
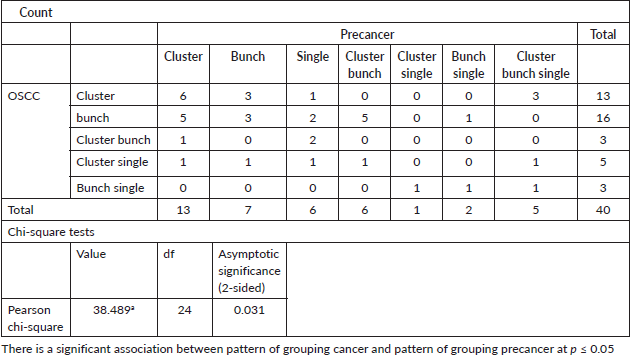
Table 5. Staining intensity of elastic fibres in cancer versus in precancer.
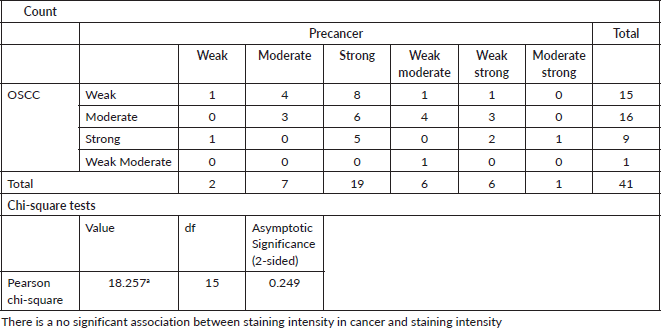
Table 6. Orientation of elastic fibres in cancer versus in precancer.
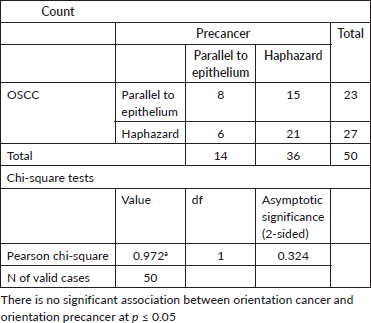
Control group
The elastic fibres in the control group showed normal long fibres, clumped fibres and fragmented fibres, but long normal-looking fibres were in the majority (Figure 1).
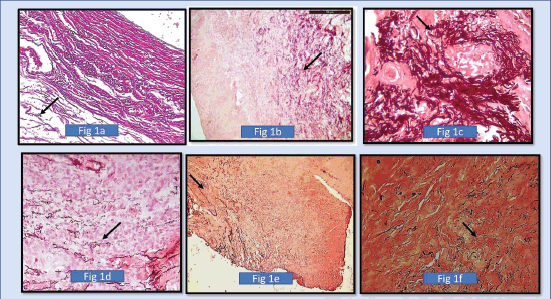
Figure 1. (a): Photomicrograph shows elastic fibres using SHIKATA’S MODIFIED ORCEIN STAIN (dark brown to black coloured stain). In the positive control group in the wall of the blood vessel, (b): Photomicrograph shows fragmented fibres (Morphology). In 10× magnification in normal oral mucous membrane using SHIKATA’S MODIFIED ORCEIN STAIN, (c): Shows photomicrograph of 40× magnification showing short, thick and wavy elastic fibres clumping of elastic fibres in well differentiated squamous cell carcinoma around tumour islands (SHIKATA’S MODIFIED ORCEIN STAIN), (d): Shows photomicrograph of moderately differentiated squamous cell carcinoma showing less density of elastic fibres (4× Magnification) (SHIKATA’S MODIFIED ORCEIN STAIN), (e): Shows photomicrograph of moderately differentiated squamous cell carcinoma showing less density of elastic fibres. (4× magnification) (SHIKATA’S MODIFIED ORCEIN STAIN), and (f): Shows photomicrograph of short, fragmented, thin straight elastic fibres in poorly differentiated squamous cell carcinoma. (40× Magnification) (SHIKATA’S MODIFIED ORCEIN STAIN).
Study group
When the density of the elastic fibres was analysed, abundant to moderate fibres were noted in well differentiated OSCC, moderately differentiated OSCC, OSMF grade 1 and 2 and scanty fibres were noted in poorly differentiated OSCC, OSMF grade 3 and OSMF grade 4; whereas in oral epithelial dysplasias the density was moderate to abundant in mild and moderate oral epithelial dysplasiascases, and scanty in severe oral epithelial dysplasias.
When morphology and pattern of grouping as evaluated in both the subgroups of study groups, the milder histopathological grades showed a combination of short, thick and wavy, whereas the higher grades showed short, thin and straight fibres. The elastic fibres were present in bunches and singly irrespective of the grades in a few cases in OSCC. Elastic fibres were fragmented or thin clusters/bunches in higher grades of the Oral Potentially Malignant disorders and in their initial grades were mostly present in bunches.
The evaluation of morphology noted short, thick, wavy fibres in both well-differentiated OSCC, Moderately differentiated OSCC, OSMF grade 1 and OSMF grade 2 and in mild oral epithelial dysplasias and moderate oral epithelial dysplasias; whereas as the grades progressed, short, thin, straight fibres were seen in poorly differentiated OSCC, OSMF grade 3, OSMF grade 4 and in severe Oral Epithelial Dysplasias. As grade progresses fibres became long/short, thin and straight (poorly differentiated OSCC and severe oral epithelial dysplasias).
The grouping pattern of the elastic fibres were evaluated as present singly, in bunches and fragmented/clustered. The well-differentiated OSCC, OSMF GRADE 1 and mild oral epithelial dysplasia showed elastic fibres singly present and in bunches. Elastic fibres showed fragmentation and were seen as short fibres in thin clusters in most of the higher grades of the cases taken in precancerous lesions/disorders group.
The findings for the staining intensity for precancerous and cancerous lesions with various grades were inconsistent.
The orientation of elastic fibres to epithelium and orientation to tumour islands is parallel in the initial grades of OSCC. Few cases of initial grades showed haphazard/perpendicular arrangement of elastic fibres. The orientation of elastic fibres to epithelium and orientation to tumour islands is haphazard and showed no significant arrangement across various grades of potentially malignant disorders/lesions (Figures 1a–e, 2a–d and 3a–c).
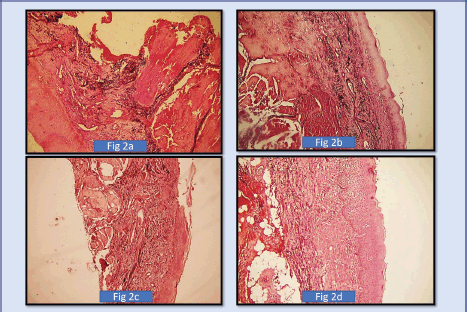
Figure 2. (a): Shows photomicrograph of short, thick, wavy elastic, fibres in OSMF Grade 1 (4× Magnification) (SHIKATA’S MODIFIED ORCEIN STAIN, (b): Shows photomicrograph of short, thick, wavy elastic fibres in OSMF Grade 2. (4× Magnification) (SHIKATA’S MODIFIED ORCEIN STAIN), (c): Shows photomicrograph of OSMF short, thin, straight, elastic fibres in OSMF Grade 3. (4× Magnification) (SHIKATA’S MODIFIED ORCEIN STAIN), (d): Shows photomicrograph of short, thin, straight, fragmented elastic fibres in OSMF Grade 4. (10× Magnification (SHIKATA’S MODIFIED ORCEIN STAIN).
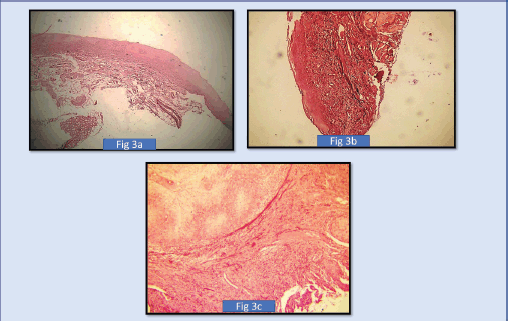
Figure 3. (a): Shows photomicrograph of short thick wavy elastic fibres, in mild oral epithelial dysplasias (4× Magnification) (SHIKATA’S Modified Orcein Stain), (b): Shows photomicrograph of moderate oral epithelial dysplasiasas short, thick wavy elastic fibres (4× Magnification) (SHIKATA’S MODIFIED ORCEIN STAIN). (c): Shows photomicrograph of long, thin, short fibres in severe oral epithelial dysplasias (4× Magnification) (SHIKATA’S MODIFIED ORCEIN STAIN).
Our study revealed that the elastic fibres were predominantly seen in the initial stages of cancer and potentially malignant disorders/lesions. Scanty or no fibres were seen in the advanced stages of cancer and potentially malignant disorders/lesions. The sparse literature, the lesser number of elastic fibres in lamina propria and the masking effect of overlying inflammatory cells could be the limiting factors in the assessment of elastic fibres in OSCC. This calls for more sensitive labelling systems for assessing elastic fibres (Figures 4–6).
When the density of elastic fibres (abundant, moderate and scanty), were correlated within the OSCC group, the statistical analysis was significant in all earlier grades as compared to advanced grades, with a significant p value < 0.05 (p = 0.002) The statistical analysis showed p value less than 0.05 (p = 0.002) which is significant with Spearman’s ratio.
When the staining intensity (strong, moderate and weak) and the orientation of elastic fibres to overlying epithelium (parallel, perpendicular/haphazard) were correlated within the OSCC group, the statistical analysis was significant in all earlier grades as compared to advanced grades (staining intensity (p = 0.025) and (p = 0.00) for orientation of elastic fibres to overlying epithelium with the Spearman’s ratio).
The statistical analysis for the morphology of elastic fibres in OSCC cases (p > 0.05)] (p = 0.183) (short, thick, wavy, long, thin and straight) and pattern of grouping (present singly, in bunches or clustered/fragmented) p value >0.05 (p = 0.869) was not significant with Spearman’s ratio (Tables 4–6) Figures 4–6.
Most of the cases of the well-differentiated OSCC showed a parallel arrangement of elastic fibres around some tumour islands and to overlying epithelium whereas in some cases of well-differentiated OSCC and higher grades showed haphazard and absence of elastic fibres except for a few cases. The staining intensity was milder in the initial grades of the OSCC and was moderate to strong in higher grades in OSCC except for a few cases.
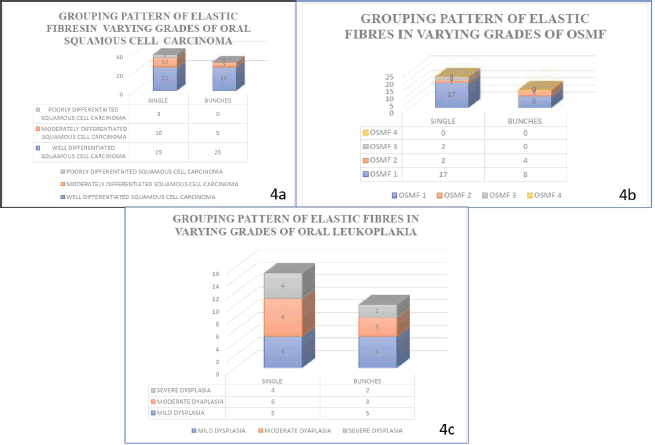
Figure 4. Graph shows the morphology of elastic fibres in various study groups.
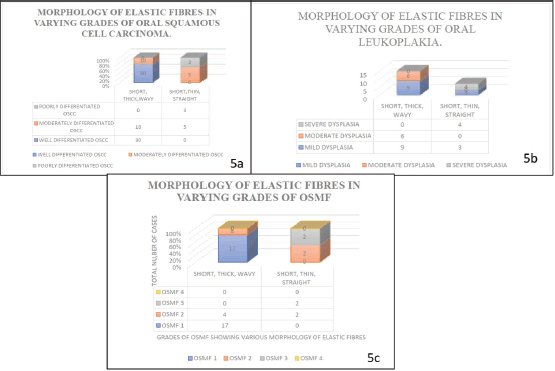
Figure 5. Graph shows grouping pattern of elastic fibres in various study groups.
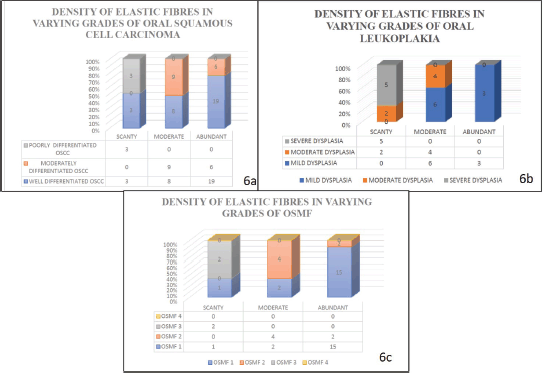
Figure 6. Graph shows the density of elastic fibres in various study groups.
The statistical analysis for the pattern of grouping (present singly, in bunches or clustered/fragmented) of elastic fibres was significant with the Spearman’s ratio p value <0.05 (p = 0.036) for oral potentially malignant lesions/disorders group. The higher grades of the oral potentially malignant disorders group showed elastic fibres in fragmented/clustered form and in thin bunches. The milder grades of the oral potentially malignant disorder/lesions group showed elastic fibres mostly in thick bunches and presented as singly.
The statistical analysis of elastic fibres for staining intensity (strong, moderate and weak) in Oral potentially malignant lesions/disorders group was not significant with Spearman’s ratio p > 0.05 (p = 0.46) The statistical analysis for density of elastic fibres in oral potentially malignant lesions/disorders group was not significant p > 0.05 (p =0.907).
The statistical analysis for the morphological patterns of elastic fibres in oral potentially malignant lesions/disorders group (OSMF and ORAL LEUKOPLAKIA) was not significant p > 0.05 (p = 0.059) no. 8). The p value was more than 0.05 for all the morphological parameters of elastic fibres (short, thick, wavy, straight, long, thick and thin) and orientation to overlying epithelium (parallel, perpendicular and haphazard). The p value >0.05 for the orientation of elastic fibres to overlying epithelium (parallel, perpendicular or haphazard) (p = 0.734) for the Oral Potentially Malignant lesions group is not significant. Out of all parameters for elastic fibres–density, morphology, staining intensity and orientation of elastic fibres to overlying epithelium, the statistical analysis shows that the p-value was more than 0.05 which is not significant in oral potentially malignant disorders/lesions.
Discussion
In this study, we employed Shikata’s modified orcein stain due to its cost-effectiveness, ease of use for novice researchers and the ready availability of required reagents. This staining method provides an accessible and efficient means of visualising elastic fibres in tissue samples.
The literature has documented therapeutic approaches that incorporate elastic molecules to enhance the healing process. These findings underscore the potential significance of elastic fibres in tissue repair and regeneration [22].
Tosios et al [23] in their investigation of elastofibromatous changes and hyperelastosis in oral mucosa, observed that the connective tissue exhibited chronic and subacute inflammation, accompanied by increased vascularity and fibrosis. They noted a progressive loss of tissue elasticity correlating with the advancing grades of the lesion. Our study of OSMF revealed similar patterns across various grades of the condition [23].
These observations collectively highlight the importance of elastic fibres in maintaining tissue integrity and function, as well as their potential role in the pathogenesis and progression of oral mucosal disorders. The alterations in elastic fibre distribution and morphology may serve as valuable indicators of disease severity and progression in conditions such as OSMF.
Nuclear pleomorphism and perineural invasion have been identified as crucial parameters in assessing cancer aggressiveness, as demonstrated by Jamadar et al [24] These findings underscore the importance of evaluating such areas for elastic fibre distribution and morphology in future studies. The relationship between these histopathological features and elastic fibre alterations could provide valuable insights into the mechanisms of tumour progression and invasion.
Kardam et al [25] conducted a comprehensive analysis of stromal fibrosis in OSCC utilising picrosirius red and Verhoeff’s Van Gieson stains. Their study revealed a notable shift in collagen fibre characteristics as the tumour progressed from well-differentiated to poorly differentiated OSCC. Specifically, they observed a predominance of thin collagen fibres exhibiting a greenish–yellow hue, while thick fibres displayed a diverse colour spectrum as the tumour grade advanced. The collagen fibres were found to be loosely packed and haphazardly arranged, suggesting a significant disruption of the ECM architecture. Our study followed similar guidelines but for elastic fibres using Shikata’s Modified Orcein Stain. The quantitative analysis of collagen and qualitative assessment of elastic fibres in Kardam et al [25]’s study yielded statistically significant results, highlighting the potential of these stromal changes as markers of tumour progression. These findings were corroborated by John and Murthy [19] and align with our observations in both precancerous and cancerous groups.
The intricate relationship between chronic inflammation and cancer development has been the subject of intense research in recent years. Tlsty and Coussens [26] provided compelling evidence that chronic inflammatory disorders significantly enhance the overall risk for cancer development. Our study builds upon this foundation, offering novel insights into the role of elastic fibres in this process.
We observed a striking absence of elastic fibres in areas of inflammation, particularly in advanced grades of the study group. This finding suggests a potential mechanistic link between the inflammatory microenvironment and the degradation of elastic fibres. The correlation between inflammation and elastic fibre alteration that we identified may represent a critical step in the progression from chronic inflammation to neoplastic transformation.
The ECM plays a pivotal role in regulating tumour behaviour and disease progression. As noted in previous studies, inappropriate synthesis or degradation of any ECM component can profoundly alter cell physiology [27]. Our research provides compelling evidence supporting this concept, demonstrating both qualitative and quantitative alterations in elastic fibres across different stages of disease progression.
Specifically, we observed progressive disorganisation and fragmentation of elastic fibres correlating with advancing disease stages. In the early stages, elastic fibres appeared relatively intact, forming an organised network within the ECM. However, as the disease progressed, we noted a marked decrease in fibre density, increased fragmentation and a loss of the normal architectural arrangement.
Quantitative analysis revealed a statistically significant reduction in elastic fibre content in advanced stages of disease compared to early stages and normal tissue. This quantitative change was accompanied by qualitative alterations, including changes in fibre thickness, continuity and spatial distribution. These observations suggest that elastic fibre degradation is not merely a consequence of disease progression but may actively contribute to the creation of a permissive microenvironment for tumour growth and invasion.
The absence of elastic fibres in areas of intense inflammation in advanced disease stages is particularly intriguing. This finding implies that inflammatory processes may directly or indirectly promote elastic fibre degradation, possibly through the action of inflammatory cell-derived proteases or by altering the expression of elastic fibre-associated proteins.
Elastic fibres play a crucial role in shaping the tumour microenvironment (TME). In the initial phases of tumour development, these fibres are abundant, contributing to the structural framework of the tissue [28–30]. However, as cancer progresses, the quantity of elastic fibres significantly decreases. In advanced cancers, the tumour stroma becomes progressively stiffened, a phenomenon that serves to protect the tumour from host defence mechanisms [31–33]. This stiffening is often associated with increased collagen deposition and other ECM modifications, leading to a more rigid microenvironment that promotes tumour survival and invasion. Matrix degradation can additionally promote malignant progression and metastasis as suggested by Erler and Weaver et al [31, 34]. These findings have significant implications for our understanding of disease progression in oral potentially malignant disorders and oral cancer. The alterations in elastic fibres could serve as a valuable biomarker for disease staging and prognosis. Moreover, targeting the processes involved in elastic fibre degradation or promoting elastic fibre preservation could represent a novel therapeutic approach.
The alterations in elastic fibres observed in our study align with several hallmarks of cancer as described by Hanahan and Weinberg [35–38]. Elastic fibre degradation may contribute to ‘sustaining proliferative signalling’ through altered mechanotransduction as suggested by Pickup et al [39], ‘activating invasion and metastasis’ by compromising tissue integrity as mentioned by Lu et al [34], and ‘tumour-promoting inflammation’ via its correlation with inflammatory processes as stated by Tlsty and Coussens and various authors. et al [26, 34, 38, 40]. The disruption of elastic fibre architecture could also play a role in ‘inducing angiogenesis’ [41] and These findings suggest that elastic fibre alterations are not merely a consequence of cancer progression but actively participate in multiple cancer hallmarks, highlighting their significance in tumour biology and potential as therapeutic targets.
They undergo dynamic changes in the accompanying tumour and results in desmoplasia and is associated with the recruitment of inflammatory cells and activation of angiogenic programmes [16].
The TME plays a crucial role in the progression of oral precancerous and cancerous lesions, with cancer-associated fibroblasts (CAFs) emerging as key players in this process. CAFs are a primary source of matrix metalloproteinases (MMPs), enzymes known to degrade ECM components, including elastic fibres according to Monteran and Erez [42]. The degradation of elastic fibres by MMPs may contribute to the altered tissue architecture observed in our study using Shikata’s modified orcein stain.
While CAFs, often identified by markers such as fibroblast activation protein α, have been implicated in promoting tumour growth, metastasis and angiogenesis across various solid tumours [36, 43], our study focuses on the direct visualisation of elastic fibres. The changes in elastic fibre distribution and morphology may reflect the degree of stromal activation and remodelling in oral precancerous and cancerous lesions.
Khalid et al [44] demonstrated an increased expression of myofibroblasts, a CAF subtype, in precancerous and cancerous oral lesions, with higher expression correlating with advanced grades of neoplasia. This finding complements our investigation of elastic fibres, as both may serve as indicators of disease progression and TME alterations.
Neoplasms undergoing malignant transformation exhibit increased architectural disarray at the invasive front of the tumour mass. At this site, there is enhanced production of matrix-remodelling enzymes and synthesis of various ECM components, particularly Type I collagen. MMPs, produced by CAFs and inflammatory cells, degrade key ECM structural components, including both collagens and elastic fibres, facilitating neoplastic progression as stated by Dineshshankar et al [18] Similar findings were observed in our study where advanced stages of potentially malignant and cancerous lesions, where elastic fibres appeared thin, short and fragmented.
Henwood [14] used the modified Shikats’s orcein staining, an old-established method for the demonstration of elastic fibres, thus providing a simple way of distinguishing collapse from fibrosis. It can be used for the demonstration of elastic fibres, HBsAg [14] and CAP in liver biopsies [16].
The density of elastic fibres was abundantly seen in the initial grades of cancerous and potentially malignant disorders group. Short, thick, wavy fibres were mostly observed in the initial grades of OSCC and potentially malignant disorders groups (ORAL LEUKOPLAKIA and OSMF) whereas long, thin, wavy with an insignificant amount of straight elastic fibres were seen in the higher grades of the cases taken for evaluation. Similar findings were seen by Dineshshankar et al [18] and John and Murthy [19] where he used Verhoef’s Van Geisson stain and Picrosirius red stain There was a significantly increased amount of fragmented/short/thin elastic fibres in poorly differentiated/Grade 3 and 4 OSMF/severe Oral Epithelial Dysplasias when compared to normal mucosa. In our study, the findings were inconsistent due to unequal numbers of cases in varying grades. Staining intensity too was inconsistent which might be due to minor changes during each lot of staining. Therefore, immunofluorescence is recommended over histochemical staining because the stain fades over a period of time.
There are various methods to visualise elastic fibres such as special stains, scanning electron microscopy and fluorescent microscopy. Since scanning electron microscopy and fluorescent microscopy are very expensive, the histochemical staining procedure is very effective and reliable [45].
Once prepared by mixing various reagents, the solution has an extended shelf life and can be stored at room temperature. Therefore, Shikata’s modified orcein stain is highly recommended. The OSMF cases in this study were clinically graded as OSMF Grade I, II, III and IV, based on the mouth opening criteria provided by Rajendran and Sivapathasundharam et al [46]. Given that OSMF exhibits extensive connective tissue fibrosis, this study also evaluated stromal elastic fibres in addition to collagen. In terms of the orientation of elastic fibres relative to the overlying epithelium and around tumour islands, the fibres were haphazardly arranged, without any significant parallel or perpendicular pattern.
Some tables showed fewer than 50 cases due to the exclusion of samples where specific parameters could not be accurately assessed. This may be due to factors such as inadequate tissue preservation, staining artefacts or absence of evaluable elastic fibres in some sections. To maintain the integrity of the analysis, only cases with clear, assessable features for each specific parameter were included in the respective tables. This approach ensures that the data presented is of high quality and reliability, even if it results in a reduced sample size for certain analyses. Future studies with larger sample sizes may help to further validate these findings.
Highlight
This would be the first study on the Description of Shikata’s modified orcein stain and its advantages in highlighting elastic fibres in OSCC and oral precancer.
The manuscript provides a valuable contribution to the scientific community by shedding light on the role of elastic fibres in the oral mucosa during the progression of precancerous conditions to cancer. The use of Shikata’s modified orcein stain enhances the precision of the analysis, making the findings particularly relevant for clinicians and researchers working in the field of oral pathology.
Recommendations and future scope
Future studies can be carried out with Shikata’s modified orcein stain to study elastic tissue in the hard tissue sections.
Future studies should aim to elucidate the molecular mechanisms underlying the observed elastic fibre alterations, particularly in the context of inflammation. Investigation of the specific proteases involved in elastic fibre degradation and the signalling pathways regulating their expression could provide valuable targets for intervention. Additionally, longitudinal studies correlating elastic fibre changes with disease outcomes could further establish their prognostic value.
Conclusion
In conclusion, this retrospective study utilising Shikata’s modified orcein stain reveals significant insights into the role of elastic fibres in oral precancerous and cancerous lesions. The density, morphology and pattern of elastic fibres correlated significantly with the grades of OSMF, oral leukoplakia and OSCC, while other parameters showed no significant correlation. In OSCC, elastic fibres exhibited haphazard arrangements and fragmentation, indicating extensive remodelling of the ECM. Moreover, the aggressiveness of tumours was linked to stromal changes, with degradation of elastic fibres at the advancing front. These qualitative and quantitative alterations suggest that elastic fibres could serve as adjunct parameters in histological grading. Ultimately, we conclude that the abundance of elastic fibres decreases as the grade of cancer and potentially malignant disorders increases, emphasising their potential role as biomarkers in tumorigenesis.
Acknowledgments
The authors would like to acknowledge lab technician Mrs. Rajshree Dahale for meticulously preserving registry data.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
1. Pindborg JJ, Reichart PA, and Smith CJ, et al (1997) Histological Typing of Cancer and Precancer of the Oral Mucosa
2. Thompson LDR (2017) Update from the 4th edition of the World Health Organisation classification of head and neck tumours: tumours of the ear Head Neck Pathol 11(1) 78–87 https://doi.org/10.1007/s12105-017-0790-5 PMID: 28247225 PMCID: 5340731
3. Ariyoshi Y, Shimahara M, and Omura K, et al (2008) Epidemiological study of malignant tumours in the oral and maxillofacial region: survey of member institutions of the Japanese Society of Oral and Maxillofacial Surgeons, 2002 Int J Clin Oncol 13(3) 220–228 https://doi.org/10.1007/s10147-007-0756-9
4. William G (2006) Shafer, Hine L. Shafer ’ s Text Book of Oral Pathology 8th edn (Delhi: Elsevier) pp 567–658
5. Tamgadge SA, Ganvir SM, and Hazarey VK, et al (2012) Oral leukoplakia: transmission electron microscopic correlation with clinical types and light microscopy Dent Res J (Isfahan) [Internet] 9(Suppl 1) S94–S104 PMID: 23814570 PMCID: 3692208
6. Langley RR and Fidler IJ (2011) The seed and soil hypothesis revisited-the role of tumour-stroma interactions in metastasis to different organs Int J Cancer 128(11) 2527–2535 https://doi.org/10.1002/ijc.26031 PMID: 21365651 PMCID: 3075088
7. van den Hooff A (1988) Stromal involvement in malignant growth Adv Cancer Res 50(C) 159–196 https://doi.org/10.1016/S0065-230X(08)60437-6 PMID: 3287842
8. Chandni S, Tamgadge S, and Tamgadge A, et al (2024) Tumour microenvironment in oral squamous cell carcinoma: special stains and scanning electron microscopic study J Microsc Ultrastruct 12(3) 148–154 https://doi.org/10.4103/jmau.jmau_19_22
9. Tuxhorn JA, Ayala GE, and Rowley DR (2001) Reactive stroma in prostate cancer progression J Urol 166(6) 2472–2483 https://doi.org/10.1016/S0022-5347(05)65620-0 PMID: 11696814
10. Timar J, Lapis K, and Fulop T, et al (1991) Interaction between elastin and tumour cell lines with different metastatic potential; in vitro and in vivo studies J Cancer Res Clin Oncol 117(3) 232–238 https://doi.org/10.1007/BF01625430
11. Sabnis SL, Kulkarni MM, and Shinde S, et al (2020) Morphological changes of extracellular matrix in different histopathological grades of oral squamous cell carcinoma Ann Rom Soc Cell Biol 24(2) 785–800
12. Kielty CM (2006) Elastic fibres in health and disease Expert Rev Mol Med 8(19) 1–23 https://doi.org/10.1017/S146239940600007X PMID: 16893474
13. Unna P (1899) Unna’s Dermatologische Preisaufgabe 2 287–288
14. Henwood T (1983) Shikata’s Orcein stain – a routine stain for liver biopsies Aust J Med Lab Sci 4(April) 77–80 [https://www.researchgate.net/publication/316510316_Shikata’s_Orcein_Stain_-_A_Routine_Stain_for_Liver_Biopsies]
15. Katsoulas N, Prodromidis G, and Nikitakis NG (2018) Lichen sclerosus of the upper lip: report of a case, utilising Shikata’s modified orcein stain, and review of the literature J Oral Maxillofac Res 9(1) 1–9 https://doi.org/10.5037/jomr.2018.9105
16. Henwood A (2003) Current applications of orcein in histochemistry. A brief review with some new observations concering influence of dye batch variation and ageing of dye solutions on staining Biotech Histochem 78(6) 303–308 https://doi.org/10.1080/10520290410001671335
17. Friedberg SH and Goldstein DJ (1969) Thermodynamics of orcein staining of elastic fibres Histochem J 1(4) 361–376 https://doi.org/10.1007/BF01003279 PMID: 4113287
18. Dineshshankar J, Ganapathy N, and Yoithapprabhunath TR, et al (2019) Morphological analysis of elastic fibres in various grades of oral squamous cell carcinoma and epithelial dysplasia using Verhoeff–Van Gieson stain Rambam Maimonides Med J 10(3) 1–6 https://doi.org/10.5041/RMMJ.10367
19. John RE and Murthy S (2016) Morphological analysis of collagen and elastic fibres in oral squamous cell carcinoma using special stains and comparison with Broder’s and Bryne’s grading systems Indian J Dent Res 27(3) 242–248 https://doi.org/10.4103/0970-9290.186242 PMID: 27411651
20. Broders AC (1941) The microscopic grading of cancer Surg Clin North Am 21 947–962
21. Rajendran R and Sundharam B (2006) Shafers Text Book of Oral Pathology 6th edn (Philadelphia: Elsevier Ltd.) pp 80–125
22. Kardam P, Mehendiratta M, and Rehani S, et al (2016) Stromal fibres in oral squamous cell carcinoma: a possible new prognostic indicator? J Oral Maxillofac Pathol 20(3) 405–412 https://doi.org/10.4103/0973-029X.190913 PMID: 27721605 PMCID: 5051288
23. Almine JF, Wise SG, and Weiss AS (2012) Elastin signalling in wound repair Birth Defects Res Part C Embryo Today Rev 96(3) 248–257 https://doi.org/10.1002/bdrc.21016
24. Tosios KI, Economou I, and Vasilopoulos NN, et al (2010) Elastofibromatous changes and hyperelastosis of the oral mucosa Head Neck Pathol 4(1) 31–36 https://doi.org/10.1007/s12105-009-0153-y PMID: 20237986 PMCID: 2825534
25. Jamadar S, Narayan T, and Shreedhar B, et al (2014) Comparative study of various grading systems in oral squamous cell carcinoma and their value in predicting lymph node metastasis Indian J Dent Res 25(3) 357–363 https://doi.org/10.4103/0970-9290.138336 PMID: 25098995
26. Tlsty TD and Coussens LM (2006) Tumour stroma and regulation of cancer development Annu Rev Pathol 1(February) 119–150 https://doi.org/10.1146/annurev.pathol.1.110304.100224
27. Peltanova B, Raudenska M, and Masarik M (2019) Effect of tumour microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review 1–24
28. Kalluri R (2016) The biology and function of fibroblasts in cancer Nat Rev Cancer [Internet] 16(9) 582–598 https://doi.org/10.1038/nrc.2016.73
29. Özdemir BC, Pentcheva-Hoang T, and Carstens JL, et al (2014) Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with diminished survival Cancer Cell 25(6) 719–734 https://doi.org/10.1016/j.ccr.2014.04.005 PMID: 24856586 PMCID: 4180632
30. Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis Nat Rev Cancer 3(6) 422–433 https://doi.org/10.1038/nrc1094 PMID: 12778132
31. Erler JT and Weaver VM (2009) Three-dimensional context regulation of metastasis Clin Exp Metastasis 26(1) 35–49 https://doi.org/10.1007/s10585-008-9209-8 PMCID: 2648515
32. Spill F, Reynolds DS, and Kamm RD, et al (2016) Impact of the physical microenvironment on tumour progression and metastasis Curr Opin Biotechnol 40 41–48 https://doi.org/10.1016/j.copbio.2016.02.007 PMID: 26938687 PMCID: 4975620
33. Lühr JJ, Alex N, and Amon L, et al (2020) Maturation of monocyte-derived DCs leads to increased cellular stiffness, higher membrane fluidity, and changed lipid composition Front Immunol 11(November) 1–18 https://doi.org/10.3389/fimmu.2020.590121
34. Lu P, Weaver VM, and Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression J Cell Biol 196(4) 395–406 https://doi.org/10.1083/jcb.201102147 PMCID: 3283993
35. Hanahan D and Weinberg RA (2011) Hallmarks of cancer tumour progression only certain paths are available to tumours as they acquire hallmarks 100(1)
36. Erez N, Truitt M, and Olson P, et al (2010) Article cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumour-promoting inflammation in an NF- k B-dependent manner Cancer Cell [Internet] 17(2) 135–147 https://doi.org/10.1016/j.ccr.2009.12.041
37. Hanahan D and Weinberg RA (2000) The hallmarks of cancer review douglas Cell 100(7) 57–70 https://doi.org/10.1016/S0092-8674(00)81683-9 PMID: 10647931
38. Hanahan D and Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumour microenvironment Cancer Cell 21(3) 309–322 https://doi.org/10.1016/j.ccr.2012.02.022 PMID: 22439926
39. Pickup MW, Mouw JK, and Weaver VM (2014) The extracellular matrix modulates the hallmarks of cancer EMBO Rep 15(12) 1243–1253 https://doi.org/10.15252/embr.201439246 PMID: 25381661 PMCID: 4264927
40. Lisa M and Coussens ZW (2002) Inflammation and cancer Nature 19(420) 860–867
41. Mouw JK, Ou G, and Weaver VM, et al (2014) Extracellular matrix assembly: a multiscale deconstruction Nat Rev Mol Cell Biol 15(12) 771–785
42. Monteran L and Erez N (2019) The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumour microenvironment Front Immunol 10(AUG) 1–15 https://doi.org/10.3389/fimmu.2019.01835
43. Qian X, Xiao F, and Chen YY, et al (2021) Computerised assessment of the tumour-stromal ratio and proposal of a novel nomogram for predicting survival in invasive breast cancer J Cancer 12(12) 3427–3438 https://doi.org/10.7150/jca.55750 PMID: 33995621 PMCID: 8120167
44. Khalid A, Siddiqui S, and Faizi N, et al (2019) Role of stromal myofibroblasts in the progression of oral lesions from dysplasia to invasive carcinoma Indian J Med Paediatr Oncol 40(4) 536–541 https://doi.org/10.4103/ijmpo.ijmpo_121_18
45. Ushiki T and Murakumo M (1991) Scanning electron microscopic studies of tissue elastin components exposed by a KOH-collagenase or simple KOH digestion method Arch Histol Cytol 54(4) 427–436 https://doi.org/10.1679/aohc.54.427 PMID: 1662055
46. Rajendran R and Sivapathasundharam BE (2006) Shafer, Hine L. Shafer’s Textbook of Oral Pathology 5th edn (New Delhi: Elsevier Ltd) pp 404–407
47. Unna PG (1890) U¨ ber die Taenzer’sche Fa¨rbung des elastischen Gewebes Monatsh Prakt Dermatoln 366–377
48. Weigert C and Workman C (1892) On the staining of the medullary sheath of nerve fibres Glasgow Med J [Internet] 38(1) 27–35 PMID: 30435727 PMCID: 5936392
49. Verhoeff FH (1908) Some new staining methods of wide applicability. Including a rapid differential stain for elastic tissue JAMA 11 876–877
50. Gieson IV (1889) Laboratory notes of technical methods for the nervous system New York Med J 50 57–60
51. Russell HK Jr (1972) A modification of Movat’s pentachrome stain Arch Pathol 94(2) 187–191 PMID: 4114784
52. Masson CLP (1929) Some histological methods. Trichrome staining and their preliminary technique J Tech Methods 12 75–90
53. Gömöri G (2000) Gomori’ s Aldehyde Fuchsin (The Jackson Laboratory Histology Lab)
54. Shikata T, Uzawa T, and Yoshiwara N, et al (1974) Staining methods of Australian antigen in paraffin sections- detection of cytoplasmic inclusion bodies Japneses J Exp Med 44 25–36
55. Brissie RM, Spicer SS, and Thompson NT (1975) The variable fine structure of elastin visualised with verhoeff’s iron hematoxylin Anat Rec 181(1) 83–94 https://doi.org/10.1002/ar.1091810107 PMID: 45879
56. Churukian CJ and Schenk EA (1976) Iron gallein elastic method – a substitute for Verhoeff’s elastic tissue stain Stain Technol 51(4) 213–217 https://doi.org/10.3109/10520297609116705 PMID: 60801
57. Krobock E, Rahbari H, and Mehregan AH (1978) Acid orcein and giemsa stain: modification of a valuable stain for dermatologic specimens J Cutan Pathol 5(1) 37–38 https://doi.org/10.1111/j.1600-0560.1978.tb00936.x PMID: 77282
58. Singh R and Gorton AWP (1989) Orcein-alcian blue staining: a new technique for demonstrating acid mucins in gastrointestinal epithelium J Clin Pathol 42(8) 881–884 https://doi.org/10.1136/jcp.42.8.881 PMID: 2475531 PMCID: 1142070
59. Chalvardjian A and Lee S (1986) HOPS: a tetrachrome stain useful in dermatopathology Am J Dermatopathol 8(2) 178–179 https://doi.org/10.1097/00000372-198604000-00017 PMID: 2424332
60. Steven P, Paulsen F, and Tillmann B (2000) Orcein-picroindigocarmine – a new multiple stain Arch Histol Cytol 63(4) 397–400 https://doi.org/10.1679/aohc.63.397 PMID: 11073070
61. Senba M (1984) Histologic staining methods for hepatitis b surface antigen (HBS AG) and interpretation of dye reactions in vitro Acta Histochemica Et Cytochemica 17 241–249 https://doi.org/10.1267/ahc.17.241
62. Salaspuro M and Sipponen P (1976) Demonstration of an intracellular copper binding protein by orcein staining in long standing cholestatic liver diseases Gut 17(10) 787–790 https://doi.org/10.1136/gut.17.10.787 PMID: 63413 PMCID: 1411183
63. Nakamura H, Kanai C, and Mizuhira V (1977) An electron stain for elastic fibres using orcein J Histochem Cytochem 25(4) 306–308 858912>https://doi.org/10.1177/25.4.858912 PMID: 858912
64. Scheuer PJ and Maggi G (1980) Hepatic fibrosis and collapse: histological distinction by orcein staining Histopathology 4(5) 487–490 https://doi.org/10.1111/j.1365-2559.1980.tb02943.x PMID: 6159296
65. Shousha S and Boxer GM (1981) Orcein as a mucin stain for gastrointestinal tissue sections J Clin Pathol 34(9) 1063–1065 https://doi.org/10.1136/jcp.34.9.1063 PMID: 6168661 PMCID: 494244
66. Dawson JF, Brochier J, and Schmitt D, et al (1984) Elastic fibres: histological correlation with orcein and a new monoclonal antibody Br J Dermatol 111 15 https://doi.org/10.1111/j.1365-2133.1984.tb05230.x
67. Pieraggi M, Nejjar I, and Julian M, et al (1986) Staining of elastic tissue by Verhoeff’s iron hematoxylin Ann Pathol 6(1) 74–77 PMID: 2424470
68. Kim JH (1987) Comparative study on orcein and victoria blue staining for the demonstration of HBsAg in human liver tissue specimens Korean J Med Technol 19(1) 179–184