Feasibility and stage at diagnosis for children with cancer: a pilot study on population-based data in a middle-income country using the Toronto childhood cancer stage guidelines
Marceli de Oliveira Santos1a*, Paulo Cesar Fernandes de Souza2, Fernanda C da Silva de Lima1, Nathalie V Balmant1, Carolina Motta3, Michele Gonçalves da Costa3, Rejane de Souza Reis4, Gemma Gatta5* and Beatriz de Camargo1*; for the Brazilian Pilot Toronto Staging Project**
1Instituto Nacional de Câncer, Rio de Janeiro, RJ 20.230-240, Brazil
2Secretaria de Estado de Saúde de Mato Grosso, Cuiabá-MT 78.049-902, Brazil
3Instituto Desiderata, Rio de Janeiro, RJ 22.280-020, Brazil
4Fundação do Câncer, Rio de Janeiro, RJ 20.231-048, Brazil
5Evaluative Epidemiology Unit, Department of Epidemiology and Data Science, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan 20133, Italy
aIn memorium
*Same contributions
**Population-based Cancer Registry of Aracaju, Sergipe, Brazil; Population-based Cancer Registry of Belo Horizonte, Minas Gerais, Brazil; Population-based Cancer Registry of Cuiabá, Mato Grosso, Brazil; Population-based Cancer Registry of Curitiba, Paraná, Brazil.
Abstract
Background: The aim was to conduct a pilot study in a middle-income country testing the use of the Toronto Childhood Cancer Staging System by Population-Based Cancer Registry (PBCR).
Methods: This study involved first the translation of the Australian pediatric cancer staging manual for 16 types of pediatric tumours. Four PBCRs from different regions of Brazil were selected for a pilot study. The study period was from 2005 to 2014, and data were collected from notification sources, including hospitals, pathological laboratories and routine medical records, and staging was done retrospectively.
Results: We identified 1,560 pediatric cancer cases diagnosed between 2005 and 2014. Notably, 94.7% met Tier 1 criteria, and 91.9% met Tier 2 criteria. The PBCR from Curitiba (south region) demonstrated higher staging feasibility (99.3% Tier 1; 96.7% Tier 2) than from Aracaju (northeast) (87.5% Tier 1; 81.3% Tier 2). Most cases had localised or regional disease (77.7%), while 14.3% were metastatic, and 8.0% could not be staged. Osteosarcoma had the highest metastasis rate (50.0%).
Conclusion: Our study demonstrates the feasibility of collecting pediatric cancer stage data from population-based registries in resource-limited settings, advancing our understanding of pediatric cancer outcomes in Brazil.
Keywords: childhood cancer, staging, population data, middle-income country
Correspondence to: Rejane de Souza Reis
Email: reis.re@gmail.com
Published: 06/11/2024
Received: 29/05/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Childhood cancer represents a small percentage of the cancer burden; thus, population-based data are less known, especially in low- and middle-income countries. Information on the stage at diagnosis is crucial for comparing outcomes across groups over time. However, collecting this information in population-based cancer registries (PBCRs) has become a challenge, as most of them do not collect this data, and the tumour/node/metastasis (TNM) system, the standard method for cancer staging in adults, is inadequate for assessing disease extent and international comparison [1].
The stage at diagnosis is an important factor in guiding the intensity of treatment that is necessary to cure’ childhood cancer. The Toronto Childhood Cancer Stage Guideline has been built to ensure an international staging system to compare stage distribution and survival among different countries and not to substitute the clinical staging used by the institution [1]. The feasibility of applying the Toronto Childhood cancer guidelines was tested in Europe, Australia and in sub-sahara Africa [2–7]. Botta et al [8] are currently conducting the Benchista project, which aims to enhance the understanding of disparities in childhood cancer survival across European regions and identify areas requiring improvements. Furthermore, it seeks to encourage the adoption of the Toronto Staging Guidelines (TGs) from PBCRs, both in Europe and beyond, for the most common solid pediatric cancers.
The overall net survival rate in Brazil, when compared to high-income countries, is lower, often attributed to delayed diagnosis as a significant factor [9–11]. The study by Allemani et al [10] analysed the 5-year survival of tumours of the central nervous system (CNS), acute lymphoblastic leukemia (ALL) and lymphomas, in children and adolescents, in different countries, including Brazil. The results showed that in Brazil, 5-year survival was below 40% in Brazil and Mexico for CNS tumours, and below 70% for ALL in Brazil, Chile, Colombia, Peru and Thailand. Five-year survival increased by more than 10% in Brazil, Bulgaria, Croatia and Poland [10]. It is noteworthy that the majority of PBCRs have incomplete data on tumour stage in cases of childhood cancer, as evidenced in a literature review conducted by experts, which analysed various aspects of pediatric cancer in Latin America, including diagnostic processes, time to diagnosis, stage at diagnosis, treatments, complications, survival programs, palliative care and end-of-life services [12].
In this context, a gap in population data becomes evident in low- to middle-income countries that could support the notion of more common advanced-stage diagnoses, as the incidence and mortality of pediatric and adolescent cancer in less developed countries vary significantly. This disparity is further exacerbated by the lack of high-quality PBCRs in these nations, resulting in a shortage of reliable information on national incidence and stages at the time of diagnosis for meaningful comparisons [13–15].
Our aim was to conduct a pilot study in a middle-income country testing the use of the Toronto Childhood Cancer Staging System by PBCR. Moreover, to describe and compare stages at diagnosis among PBCR in four different regions in Brazil with European, Australia and Africa studies.
Material and methods
We first had permission to translate the staging manual done by the Australian group [16, 17]. A personal meeting among pediatric oncologist, cancer registries and epidemiologist was organised in April 2019. We invited several Brazilian PBCR, and four of 4 different regions showed interest to participated (Northeast (Aracaju), Southeast (Belo Horizonte), South (Curitiba) and Central-West (Cuiaba)) for a pilot study. Local training was prepared and done by one of the members of staging manual translation (NB/BDC).
The study included different periods of incidence within the years from 2005 to 2014 (Aracaju: 2005–2013; Belo Horizonte 2005–2013; Cuiaba: 2005–2011 and Curitiba 2005–2014). Cases were selected by PBCR – a database with selected cases including the notification sources were done. Notification sources include (specialised hospitals, other hospitals, pathological labs and Mortality Information System) as described in Figure 1. Data collection included demographic variables (sex, residence and age at diagnosis) information on examinations for the diagnosis (microscopic or clinical) cancer site and morphology codified by the ICD-O3, according to the TG, Information was extracted from routine medical records and pathological reports. The staging was done retrospectively for all cases according to Toronto Guidelines that include tier 1 and tier 2 [1] (Supplementary Table).
Results
Feasibility
1,560 cases younger than 19 years were identified in the four PBCR during the period between 2005 and 2014. It was not possible to retrieve medical records for 695 cases – most of them in non-specialised departments; therefore, it was possible to perform stage in 866 cases (Table 1). The median collection time was 13 minutes and 42 seconds.
Of the 866 cases, 820 (94.7%) had sufficient information in the medical records possible to apply the stage according to Tier 1 criteria and 796 (91.9%) according to Tier 2 criteria. The PBCR of Curitiba was more feasibility in staging all tumours (99.3 Tier 1; 96.7 Tier 2), and Aracaju had a lower percentage (87.5 Tier 1; 81.3 Tier 2) (Table 2).
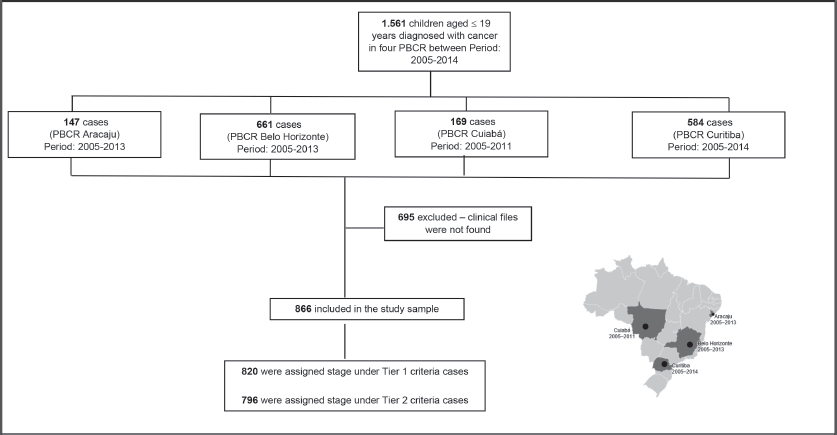
Figure 1. Flow diagram of casuistic selection
For the haematologic tumours, the acute lymphoblastic leukaemia cases stage was reconstructed slightly more (Tier 1 96%, tier 2 91%). Among solid tumours, neuroblastoma and germ cell tumour of testis and ovary stage at diagnosis were totally reconstructed according to Tier 1 and 2 (Table 2), 673 (77.7%) of cases in the study had localised or regional disease at diagnosis, 124 (14.3%) were diagnosed with metastatic cancer and the remaining 69 (8.0%) cases were not possible to perform staging (Table 3). Stage distribution by tumour can be seen in Table 3. Osteosarcoma has the most frequency of advanced disease with 45.0% of new cases diagnosed with metastases at diagnosis (Table 3). On the contrary, medulloblastoma (5%), ependymoma (17%), ovarian germ cell tumour (20%) and rhabdomyosarcoma (21%) were the solid tumours with the lowest percentages of the metastatic stage at diagnosis. Important to note that also in Brazil, no metastatic retinoblastoma was reported. Regarding to neuroblastoma and Wilms tumour comparison with the Australian and European groups can be seen in Table 4. Both with a metastatic lesions higher in Brazilian children versus the Australian and European ones.
Table 1. Characteristics of the study cohort.
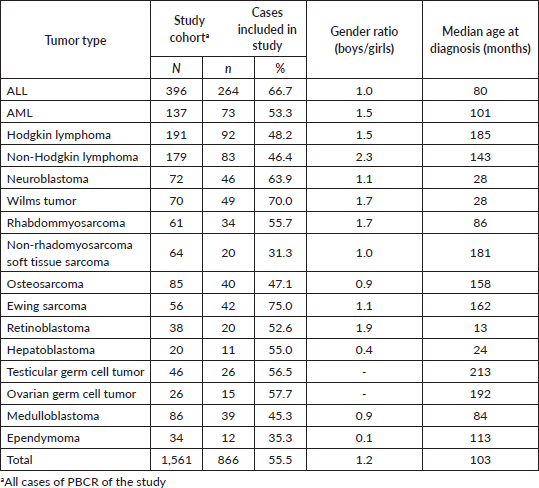
Table 2. Cases of childhood cancer could be staged according to Tier 1 and Tier 2 criteria of the Toronto guidelines by Brazilian registry.
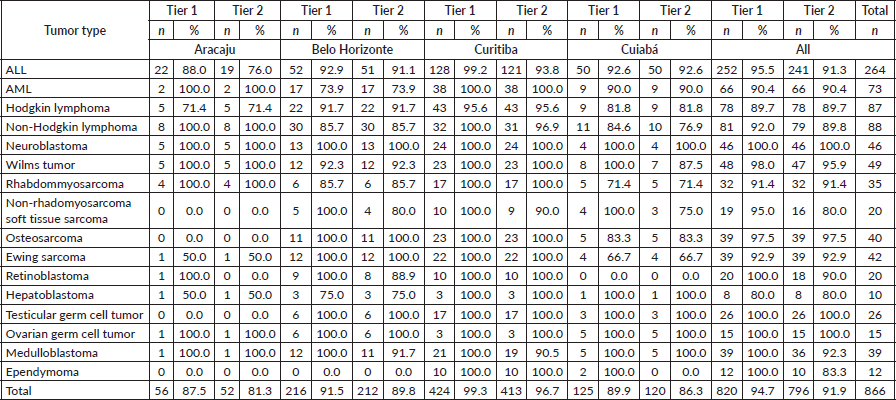
Table 3. Comparisons of stage distribution between Australia and Brazil.
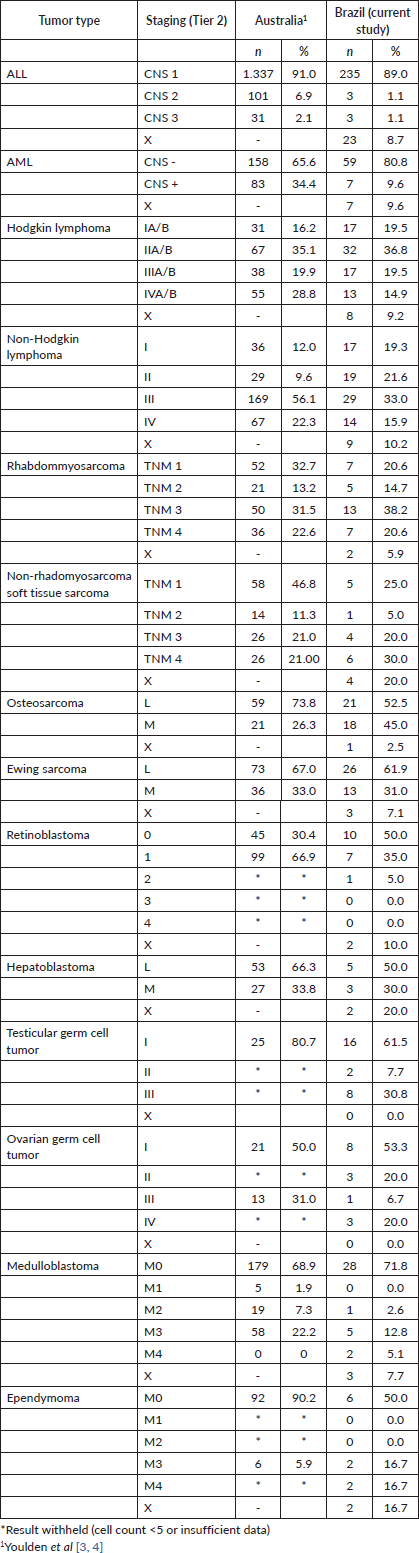
Table 4. Neuroblastoma and Wilms tumours, comparisons of stage distribution between Australia, European and Brazilian registries.
Discussion
The stage of disease represented a major prognostic variable. The type of stage varies among pediatric oncology institutions according to which treatment protocol is used. It is extremely necessary to have a uniform tool to compare international staging and survival at the population level. There are none population-based study regarding stage at diagnosis for childhood cancer in Brazil. This pilot study demonstrates that the Toronto guidelines is feasible in a middle-income country as Brazil. Completeness of data was obtained by medical records in 94.7% Tier 1 and 91.9% Tier 2 of patients with slight differences between PBCR in different geographic regions in Brazil. The study provides also a comparison with the Australian and the European PBCRs showing minor differences. At the time of this study, the European study only provided staging information for neuroblastoma and Wilms’ tumours.
It is important to highlight that notably, published data regarding the stages and survival of pediatric tumours primarily stem from clinical trials, exhibiting variations in selection criteria, in contrast to PBCR data [18, 19]. Given this, the findings of our study are nationally representative due to the standardization of stage information at the time of diagnosis through the utilization of the Toronto consensus, as well as the potential for data comparability across countries.
Diagnosis delay in childhood cancer is one of the predominant causes of disseminated malignancies [20]. It is described in low-middle-income countries that advanced disease is more frequent [21]. In the early 80´s, advanced disease was a major issue in Brazil and professional groups approached medical schools with lectures and local papers. Lay people were reached through newspaper, television, etc. and significant improvement has been noticed [22]. Early diagnosis of cancer is important to allow the opportunity for effective treatment, with fewer side effects and improved survival. Early diagnosis program has been done in Brazil being the biggest program supported by Ronald McDonald Institute since 2008 and significant improvement has been seen in some regions [23]. Several campaigns for early diagnosis of retinoblastoma were implanted [24]. Actually, in our study, we cannot see metastatic retinoblastoma, which was the case in the early 1980’ years.
Surprisingly we found a similar proportion of localised and advanced disease in the majority of tumours comparing with European and Australian data [3, 5]. Interestingly, a study conducted in three African countries demonstrated the feasibility of assigning stage at diagnosis in over 80% of cases based on the Toronto guidelines. However, a large proportion of half of the cases (52%) presented with advanced disease stage. It is worth noting that stage information at the time of diagnosis emerged as a significant predictive variable, as cases with an advanced stage for each of the three analysed tumours (non-Hodgkin lymphoma, retinoblastoma or Wilms tumour) exhibited poorer survival rates [7]. This suggests difficulties in accessing to specialised centers in the African region studied.
In our study, children with ALL were classified as CNS1 in 89% similarly as the Australian data (91%). On the contrary for acute myeloid leukemia (AML), CNS-staged children were higher in Brazil (81%) as in Australia (66%). The extent of CNS involvement can be difficult to know by registrars and CNS involvement is more frequent in the AML. However, delay diagnosis could be not higher than in high-income countries and was not associated with morbidity and early mortality in children with acute leukemia when treated in a specialised pediatric oncology unit in Northeast Brazil [9]. Access to specialised centers and family education may affect the early diagnosis. In a recent 5-year net survival study from Concord 3, the survival rates for all leukemia combined in the 0–14 years age group ranged from 48% (95% CI 26–70) in India to 91% (95% CI 84–98) in Puerto Rico. Among adolescents (15–19 years), survival varied from 24% (95% CI 11–38) in Colombia to 85% (95% CI 75–95) in Denmark. Survival was higher in the 0–14 age group compared to the 15–19 age group. Specifically, in Brazil, the 5-year net survival rates for all combined leukemia, as well as for the subtypes of ALL and AML, were 67.3% (95% CI 59–75), 72.8% (95% CI 64–81) and 42.9% (95% CI 22–63), respectively [25]. Gatta et al [26] found that in high-income European countries, more than 80% of children diagnosed with ALL manage to survive for 5 years after receiving the diagnosis.
The Brazilian Renal tumour group has shown improvement of survival but is still inferior to high-income countries. The mean tumour volume at diagnosis among Brazilian children was not higher than in other countries. Unfortunately, this population represented only 10% of the estimated number of cases. Toxicity related to treatment was higher [27]. It is well known that access to centralised expertise for radiology and pathology review can influence stage distributions and treatment. It is the aim of the next Brazilian group participation on the UMBRELLA Renal Tumour Study [28].
Significant differences of localised and advanced disease were seen in patients with osteosarcoma. We observed a higher proportion to children with metastases than in Australia data. In a study of patients treated in a Brazilian cooperative group, metastases at diagnosis were present in 21% of patients reflecting advanced disease at diagnosis. The time from onset of symptoms to diagnosis did not correlate with the presence of metastases, tumour size or survival suggesting that the stage of disease at presentation depends more on biological aspects of the tumour than late diagnosis [29]. This data confirms that is necessary biological studies in patients with osteosarcoma in Brazil, as the TG-2 suggested. Actually, Gupta et al [1] suggested to collect other clinical variables, than stage at diagnosis, such as the so called not stage prognosticators.
Regarding the application of the TGs, our results have revealed a significant proportion of cases with an unknown stage, indicating a lack of comprehensive information in medical records. This gap may be attributed to the need for enhancing the quality of information recorded in patient charts or emphasising closer collaboration with pediatric oncologists to improve the clinical data collection process. Furthermore, in a recent study conducted by Liu et al [30], it was demonstrated that the feasibility of applying these guidelines in Sub-Saharan African countries resulted in staging being attainable in 71% of cases, with variations ranging from 53% to 83% for specific cancer types. This level of accuracy is closely approximated that observed in high-income countries, particularly in the context of solid tumours. This finding represents a significant stride toward understanding the challenges posed by pediatric cancer in low- and middle-income countries [30].
Another study on the feasibility of implementing the Toronto guidelines in seven pediatric oncology units in Sub-Saharan Africa from 2017 to 2019 revealed that it was possible to assign the stage at the time of diagnosis in 89% (1,772) of eligible cases for 11 types of cancer, except for leukemia and CNS tumours [31]. The lack of information in medical records prevented it at Tier 2, unlike our results that demonstrated the possibility of staging cases in both Tier 1 and Tier 2. Additionally, the percentage of metastatic cases in our study was 14.3% and 77.7% [31].
Youlden et al [32] reported that the distribution of stages has remained stable for most types of childhood cancer in Australia over the past two decades, with the exception of retinoblastoma and hepatoblastoma. Furthermore, significant improvements in 5-year survival rates were observed, particularly among recently diagnosed children, including those with advanced solid tumours.
The World Health Organization [33] launched the Global Initiative for Childhood Cancer in 2018. The purpose of this initiative is to provide support to governments in establishing and sustaining high-quality national childhood cancer treatment programs. The established global objective is to achieve a minimum survival rate of 60% for children and adolescents aged 0 to 19 with cancer by the year 2030. To effectively monitor and evaluate the adopted actions, the need for a high-quality information system and well-structured surveillance of childhood cancer through PBCRs is imperative [34].
This is the first population-based data with a standard staging system and despite the small numbers of cases stage distribution is comparable to other countries among most of the diseases. Stage at diagnosis influences overall and event-free survival, cost of treatment and late effects. Survival rates in Brazil are inferior comparing with high-income countries and advanced disease may not be the only reason [10, 25]. Treatment in a specialised center with a multidisciplinary team and the development of a network of hospitals/departments, is extremely recommend improving survival.
Strengths and limitations
The strengths of our study included the use of PBCR data and the comparability of disease extent estimates at the population level.
The main limitations of this study include its retrospective nature and the small number of cases, limited to capital cities where PBCRs are available. Part of the data collection occurred during the COVID-19 pandemic, which led to the unavailability of several patient records. Another significant limitation is the lack of follow-up data, preventing the calculation of overall survival. Additionally, 695 cases were excluded due to missing medical records, which may affect the comprehensiveness of the findings. The low incidence of certain tumour types in specific regions could also introduce bias, limiting the generalizability of the results.
It was very important for education among cancer registries and despite the small number of cases; it suggests improvement in reconstructing stage disease in our country.
Conclusion
Our results demonstrate that it is feasible to collect information on the stage at diagnosis of children diagnosed with cancer from PBCRs, both at Tier 1 and Tier 2, even in resource-limited settings. This represents a significant advancement in the pursuit of a deeper understanding of adverse outcomes in pediatric cancer in Brazil.
Acknowledgments
Brazilian Pilot Toronto Staging Project group members: Population-based Cancer Registry of Aracaju, Sergipe, Brazil: Carlos Anselmo Lima and José Erinaldo Lôbo de Oliveira; Population-based Cancer Registry of Belo Horizonte, Minas Gerais, Brazil: Gil Patrus Mundim Pena, Claudina Agnese Casale and Silvania Lourenço Ribeiro; Population-based Cancer Registry of Cuiabá, Mato Grosso, Brazil: Damary Ormond, Katia Sirley Belchior Marinho, Maria José Lemes de Oliveira Sales and Sonia Pio; Population-based Cancer Registry of Curitiba, Paraná, Brazil: Cyntia Asturian Laporte and Dulce Meri Blitzkow. Eduardo Ribeiro Lima, Oncologist Pediatric, Hospital Baleia, Belo Horizonte/MG; Joaquim Caetano de Aguirre Neto, Oncologist Pediatric, Hospital Santa Casa de Belo Horizonte, Belo Horizonte/MG; Mara Albonei Dudeque Pianovski, Oncologist Pediatric, Hospital Erasto Gaertner (Hospital Erastinho), Curitiba/PR.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
Financial support from Desiderata Institute was received for data collection and analysis (Nathalie Balmant, José Erinaldo Lôbo de Oliveira, and Fernanda Cristina da Silva de Lima).
References
1. Gupta S, Aitken JF, and Bartels U, et al (2016) Paediatric cancer stage in population-based cancer registries: the Toronto consensus principles and guidelines Lancet Oncol 17(4) e163–e172 https://doi.org/10.1016/S1470-2045(15)00539-2 PMID: 27300676
2. Aitken JF, Youlden DR, and Moore AS, et al (2018) Assessing the feasibility and validity of the Toronto childhood cancer stage guidelines: a population-based registry study Lancet Child Adolesc Health 2(3) 173–179 https://doi.org/10.1016/S2352-4642(18)30023-3 PMID: 30169253
3. Youlden DR, Gupta S, and Frazier AL, et al (2019) Stage at diagnosis for children with blood cancers in Australia: application of the Toronto paediatric cancer stage guidelines in a population-based national childhood cancer registry Pediatr Blood Cancer 66(6) e27683 https://doi.org/10.1002/pbc.27683 PMID: 30803139
4. Youlden DR, Frazier AL, and Gupta S, et al (2019) Stage at diagnosis for childhood solid cancers in Australia: a population-based study Cancer Epidemiol 59 208–214 https://doi.org/10.1016/j.canep.2019.02.013 PMID: 30831553
5. Gatta G, Botta L, and Capocaccia R, et al (2021) Staging childhood cancers in Europe: application of the Toronto stage principles for neuroblastoma and Wilms tumour. The JARC pilot study Pediatr Blood Cancer 68(9) e29020 https://doi.org/10.1002/pbc.29020 PMID: 34114308
6. Sacerdote C, Mosso ML, and Alessi D, et al (2020) An application of the Toronto childhood cancer stage guidelines in three population-based cancer registries: the case of central nervous tumors Pediatr Blood Cancer 67(6) e28303 https://doi.org/10.1002/pbc.28303 PMID: 32301558
7. Parkin DM, Youlden DR, and Chitsike I, et al (2021) Stage at diagnosis and survival by stage for the leading childhood cancers in three populations of sub-Saharan Africa Int J Cancer 148(11) 2685–2691 https://doi.org/10.1002/ijc.33468 PMID: 33433927
8. Botta L, Gatta G, and Didonè F, et al (2022) International benchmarking of childhood cancer survival by stage at diagnosis: the BENCHISTA project protocol PLoS One 17(11) e0276997 https://doi.org/10.1371/journal.pone.0276997 PMID: 36327231 PMCID: 9632762
9. Lins MM, Amorim M, and Vilela P, et al (2012) Delayed diagnosis of leukemia and association with morbid-mortality in children in Pernambuco, Brazil J Pediatr Hematol Oncol 34(7) e271–276 https://doi.org/10.1097/MPH.0b013e3182580bea PMID: 22935656
10. Allemani C, Matsuda T, and Di Carlo V, et al (2018) Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries Lancet Lond Engl 391(10125) 1023–1075 https://doi.org/10.1016/S0140-6736(17)33326-3
11. Balmant NV, de Paula Silva N, and de O Santos M, et al (2019) Delays in the health care system for children, adolescents, and young adults with bone tumors in Brazil J Pediatr (Rio J) 95(6) 744–751 https://doi.org/10.1016/j.jped.2018.07.003
12. Guzman CPC, Cordoba MA, and Godoy N, et al (2021) Childhood cancer in Latin America: from detection to palliative care and survivorship Cancer Epidemiol 71(Pt B) 101837 https://doi.org/10.1016/j.canep.2020.101837
13. Lam CG, Howard SC, and Bouffet E, et al (2019) Science and health for all children with cancer Science 363(6432) 1182–1186 https://doi.org/10.1126/science.aaw4892 PMID: 30872518
14. Bhakta N, Force LM, and Allemani C, et al (2019) Childhood cancer burden: a review of global estimates Lancet Oncol 20(1) e42–e53 https://doi.org/10.1016/S1470-2045(18)30761-7 PMID: 30614477
15. Piñeros M, Mery L, and Soerjomataram I, et al (2021) Scaling up the surveillance of childhood cancer: a global roadmap J Natl Cancer Inst 113(1) 9–15 https://doi.org/10.1093/jnci/djaa069 PMCID: 7781445
16. Aitken J, Youlden DR, and Moore AS, et al (2017) Childhood Cancer Staging for Population Registries According to the Toronto Childhood Cancer Stage Guidelines [Internet] [https://cancerqld.blob.core.windows.net/site/content/uploads/2018/12/Childhood-cancer-staging-for-population-registries.pdf] Date accessed: 02/03/23
17. Aitken JF, Youlden DR, and Moore AS, et al (2019) Estadiamento dos tumores Malignos na Infância para Registros de Câncer de acordo com o Toronto Childhood Cancer Stage Guidelines [Internet] (Rio de Janeiro) p 64 [https://desiderata.org.br/production/content/uploads/2020/04/4bbe96342944fa2f4763e0223532c282.pdf] Date accessed: 05/02/23
18. Brandalise SR, Viana MB, and Pinheiro VRP, et al (2016) Shorter maintenance therapy in childhood acute lymphoblastic leukemia: the experience of the prospective, randomized Brazilian GBTLI ALL-93 protocol Front Pediatr 4 110 https://doi.org/10.3389/fped.2016.00110 PMID: 27800472 PMCID: 5066157
19. Ghasemi DR, Fleischhack G, and Milde T, et al (2022) The current landscape of targeted clinical trials in non-WNT/Non-SHH medulloblastoma Cancers 14(3) 679 https://doi.org/10.3390/cancers14030679 PMID: 35158947 PMCID: 8833659
20. Verma N and Bhattacharya S (2020) Time to diagnosis and treatment of childhood cancer Indian J Pediatr [Internet] 87(8) 641–643 Date accessed: 11/04/24 https://doi.org/10.1007/s12098-020-03217-y PMID: 32056193
21. Cotache‐Condor C, Kantety V, and Grimm A, et al (2023) Determinants of delayed childhood cancer care in low‐ and middle‐income countries: a systematic review Pediatr Blood Cancer [Internet] 70(3) e30175 Date accessed: 11/04/24 https://doi.org/10.1002/pbc.30175
22. de Camargo B, de Andrea ML, and Franco EL (1987) Catching up with history: treatment of Wilms’ tumor in a developing country Med Pediatr Oncol 15(5) 270–276 https://doi.org/10.1002/mpo.2950150510 PMID: 2821370
23. Instituto Ronald McDonald (2023) Programa Diagnóstico Precoce: 10 anos trabalhando por resultados efetivos [Internet] https://institutoronald.org.br/diagnostico-precoce/ Date accessed: 05/10/23
24. TUCCA (Associação para Crianças e Adolescentes com Câncer) (2023) Campanha Nacional de Conscientização e Incentivo ao Diagnóstico Precoce do Retinoblastoma [Internet] https://tucca.org.br/retinoblastoma/ Date accessed: 05/10/23
25. Ssenyonga N, Stiller C, and Nakata K, et al (2022) Worldwide trends in population-based survival for children, adolescents, and young adults diagnosed with leukaemia, by subtype, during 2000-14 (CONCORD-3): analysis of individual data from 258 cancer registries in 61 countries Lancet Child Adolesc Health 6(6) 409–431 https://doi.org/10.1016/S2352-4642(22)00095-5 PMID: 35468327
26. Gatta G, Botta L, and Rossi S, et al (2014) Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5 – a population-based study Lancet Oncol 15(1) 35–47 https://doi.org/10.1016/S1470-2045(13)70548-5
27. de Aguirre-Neto JC, de Camargo B, and van Tinteren H, et al (2022) International comparisons of clinical demographics and outcomes in the International Society of Pediatric Oncology Wilms Tumor 2001 Trial and Study JCO Glob Oncol [Internet] 8 e2100425 Date accessed: 02/04/24 https://doi.org/10.1200/GO.21.00425 PMID: 35537105 PMCID: 9126524
28. Van den Heuvel-Eibrink MM, Hol JA, and Pritchard-Jones K, et al (2017) Position paper: rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol Nat Rev Urol 14(12) 743–752 https://doi.org/10.1038/nrurol.2017.163 PMID: 29089605
29. Petrilli AS, de Camargo B, and Filho VO, et al (2006) Results of the Brazilian osteosarcoma treatment group studies III and IV: prognostic factors and impact on survival J Clin Oncol Off J Am Soc Clin Oncol 24(7) 1161–1168 https://doi.org/10.1200/JCO.2005.03.5352
30. Liu B, Abraham N, and Chitsike I, et al (2023) Enhancing information on stage at diagnosis for childhood cancer in Africa Pediatr Blood Cancer 70(10) e30555 https://doi.org/10.1002/pbc.30555 PMID: 37432023
31. Mallon B, Kaboré R, and Couitchere L, et al (2023) The feasibility of implementing Toronto childhood cancer stage guidelines and estimating the impact on outcome for childhood cancers in seven pediatric oncology units in sub-Saharan Africa. A study from the Franco-African Pediatric Oncology Group Pediatr Blood Cancer 70(12) e30664 https://doi.org/10.1002/pbc.30664 PMID: 37732944
32. Youlden DR, Baade PD, and Frazier AL, et al (2023) Temporal changes in childhood cancer incidence and survival by stage at diagnosis in Australia, 2000-2017 Acta Oncol Stockh Swed 62(10) 1256–1264 https://doi.org/10.1080/0284186X.2023.2251668
33. World Health Organization WHO Global Initiative For Childhood Cancer: An Overview [Internet] (Geneva: WHO) [https://www.who.int/docs/default-source/documents/health-topics/cancer/who-childhood-cancer-overview-booklet.pdf] Date accessed: 25/10/23
34. Piñeros M, Znaor A, and Mery L, et al (2017) A global cancer surveillance framework within noncommunicable disease surveillance: making the case for population-based cancer registries Epidemiol Rev 39(1) 161–169 https://doi.org/10.1093/epirev/mxx003 PMID: 28472440
Supplementary information
Supplementary Table 1. Summary adapted of the Toronto guidelines for the staging of pediatric cancer.