Soluble epidermal growth factor receptor (sEGFR) and carcinoembryonic antigen (CEA) concentration in patients with non-small cell lung cancer: correlation with survival after erlotinib and gefitinib treatment
I Kappers1, M A Vollebergh2, H van Tinteren3, C M Korse4, L L Nieuwenhuis1, J M G Bonfrer4, H M Klomp1, N van Zandwijk2 and M M van den Heuvel2
Department of Surgery1, Department of Thoracic Oncology2, Department of Statistics3, Department of Clinical Chemistry4, The Netherlands Cancer Institute–Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands
Correspondence to: I Kappers. Email: i.kappers@nki.nl
Abstract
Background: In patients with non-small cell lung cancer (NSCLC), a higher response rate can be achieved with epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) when selection for therapy is guided by mutation analysis or gene amplification. However, both tests are complex and require tumour tissue. Simple methods to identify responders prior to EGFR-TKI treatment are urgently needed. This study aimed to define the relation between serum sEGFR levels, carcinoembryonic antigen (CEA) and survival in NSCLC patients treated with EGFR-TKIs.
Methods: Patients with stage III/IV NSCLC treated with gefitinib or erlotinib between July 2002 and December 2005 were reviewed. Levels of serum soluble EGFR (sEGFR) were determined by a sandwich quantitative enzyme-linked immunosorbent assay. A chemiluminescence immunoassay was used for CEA. The relation between sEGFR and survival was investigated.
Results: One hundred and two NSCLC patients, mainly stage IV (80%), were identified. Mean sEGFR at baseline was 55.9 μg/l (range 35.3–74.5 μg/l). The median CEA level was 11.1 μg/l (range <1.0–2938.0 μg/l). Median overall survival was 5.2 months (range 1–52 months). Decreasing log CEA values (HR 1.51, 95% CI 1.11–2.04, multivariate analysis) and increasing sEGFR values (HR 0.96, 95% CI 0.93–0.99, multivariate analysis) were both independently associated with prolonged survival. Higher levels of pre-treatment sEGFR were associated with lower risk of progressive disease within three months (p=0.04).
Conclusions: Both baseline sEGFR and CEA levels in NSCLC patients receiving EGFR-TKIs showed a significant correlation with survival. To distinguish whether these factors have a predictive or a prognostic value, validation is warranted in an independent patient series containing a control arm without EGFR-TKI treatment.
Background
The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is abnormally activated in different types of epithelial malignancies. A constitutively activated EGFR can lead to malignant transformation of the cell. It was shown 20 years ago that blocking of the EGFR could inhibit cell proliferation in these transformed cells [1]. Since these first observations various drugs have been developed that target either the extra-cellular domain or the intracellular tyrosine kinase domain of the EGFR. Especially, drugs of the latter category, small molecule adenosine triphosphate-competitive inhibitors of the receptor's tyrosine kinase (EGFR-TKIs), such as erlotinib and gefitinib, have proven their efficacy in the treatment of non-small cell lung cancer (NSCLC) [2–4]. However, response rates of erlotinib and gefitinib in unselected patient populations are low, and selection of patients is warranted to increase response rates to a more satisfying level. A response rate of 30% can be achieved when selection of patients is based on their phenotype (female gender, non-smoking status, Asian origin, adeno- or bronchioloalveolar carcinoma) [5–9]. This can be increased up to 70% when selection is based on EGFR mutation or FISH analysis [10–18]. However, for these assays availability of tumour tissue is a prerequisite, while this is frequently not at hand in advanced NSCLC. More simple and accessible predictors of response are warranted.
Recently, soluble EGFR (sEGFR) was recognized as a potential screening tool for epithelial cancer [19,20]. SEGFR is a proteolytically cleaved form of the extra-cellular domain of the EGFR and can be measured directly in the serum [21,22]. The plausibility of sEGFR being a surrogate marker for response to treatment with an EGFR-TKI is based on the hypothesis that the level of sEGFR reflects the absolute number of activated receptors, susceptible to inhibition [23]. A decrease in sEGFR during treatment with gefitinib has been recognized to correlate with disease control in patients with NSCLC [24]. However, the role of baseline sEGFR as a predictive marker for response and survival in clinical practice is still uncertain.
The conventional tumour marker carcinoembryogenic antigen (CEA) is a member of the immunoglobulin supergene family, a cell surface adhesion protein, and it is thought to play a role in cell-to-cell adhesion(22). Since there is evidence that elevated pre-treatment levels of CEA are also predictive for response and outcome after the EGFR-TKI treatment, independent of histological subtype, we decided to study both baseline sEGFR and CEA levels in relation to survival after the EGFR-TKI treatment [25].
Patients and methods
Patients selection and study design
Between July 2002 and December 2005, patients with advanced non-small cell lung cancer, not responding to conventional chemotherapy or unable to receive chemotherapy due to poor medical condition, were offered treatment with gefitinib (Iressa®) or erlotinib (Tarceva®) as part of the Expanded Access Programme, on a compassionate use basis. Consecutive patients who were treated for more than 14 days were identified and enrolled in this study if pre-treatment serum was available for sEGFR analysis. The final sample size was determined according to the number of available patient serum samples. Hospital records were retrospectively reviewed for age, gender, race, smoking status, histological subtype, stage, side effects and toxicity of the EGFR-TKI treatment and best overall response to EGFR-TKI. Informed consent was obtained from all patients. For design and report of this study, the REMARK guidelines were followed [26].
Patients receiving gefitinib were treated with a daily dose of 250 mg. In case of unacceptable or severe (grades 3–4) toxicity, the treatment with gefitinib was interrupted. Erlotinib was administered in daily doses of 150 mg. Dose changes of 50 mg were possible in case of unacceptable toxicity. Adverse events were assessed according to the National Cancer Institute—Common Toxicity Criteria version 2. Treatment of gefitinib or erlotinib was continued until disease progression or the occurrence of a serious adverse event.
Assessment of sEGFR and CEA levels
Blood samples had to be collected within two months before start of treatment with EGFR-TKIs. Serum was stored at -30˚C. Concentration levels of the EGFR-extra-cellular-binding domain were determined by a sandwich quantitative enzyme-linked immunosorbent assay (EGFR Microtiter ELISA; Oncogene Science, Cambridge, MA) according to the manufacturer's instructions. The normal range is 45–78 μg/l as described previously [27]. Carcinoembryonic antigen was measured on the E170 analyzer, which is based on chemiluminescent immunometric technology (Roche Diagnostics, Mannheim, Germany) [28].
Response assessment and statistical analysis
Correlation among sEGFR, CEA and age were studied using Pearson correlation analysis. Associations between sEGFR, CEA, gender, stage (III, IV), smoking status (smoker, non-smoker) and histology (adenocarcinoma, squamous cell carcinoma and large cell undifferentiated) were investigated by means of the Student's t test or generalized linear regression.
Overall survival was calculated using the Kaplan–Meier method, from the first day of treatment with the EGFR-TKI to the date of death. Differences in survival between subgroups of patients were determined using the log rank test. Univariate analysis (Cox proportional hazard regression analysis) was used to detect associations between sEGFR and CEA levels and survival. Furthermore, age, gender, smoking status, tumour stage, histology and treatment drug were investigated. The assumptions of linearity and proportional hazards for sEGFR and CEA were checked by means of Martingale residuals and scaled Schoenfeld residuals [29,30]. Continuous variables (age, sEGFR and logCEA) were tested for possible non-linear associations (violence of the proportional hazards assumptions). To present Kaplan–Meier plots for sEGFR and logCEA, a cut-off was used to divide these factors into two separate groups (i.e. high vs low). A spline function through the Martingale residuals of sEGFR and logCEA was used to determine possible cut-off values, i.e., the concentration of sEGFR or CEA, where the line crossed through zero of the Martingale residuals, was used as the cut-off. Variables achieving a probability value of less than 0.10 in the univariate analysis as well as pre-operative factors considered relevant in the available literature [31–33] were introduced in a multivariate stepwise proportional hazard analysis to identify variables significantly associated with survival. p-values < 0.05 were considered statistically significant.
Response evaluation was performed using computed tomography
(CT) according to the Response
Evaluation Criteria In Solid Tumors (RECIST) [34].
Response measurement at fixed intervals was not available for every patient.
The occurrence of early progressive disease (PD) (within three months) was
investigated to analyze the relation between (non-) response and sEGFR and/or
CEA levels. Associations between high or low sEGFR and/or CEA levels, and early
occurrence of PD were tested using non-parametric tests. For this purpose,
sEFGR and log CEA were dichotomized by the cut-off value described above.
Results
Over a 3.5 years period, 145 patients with advanced non-small cell lung cancer were treated with gefitinib or erlotinib. Of these, 102 patients with available serum samples were eligible, 54 men and 48 women, with a mean age of 59 years (95% CI 57–61 years). Patients' characteristics are shown in Table 1. The median follow-up was 161 days (range 17–1581 days). EGFR mutation status was assessed in 13 patients, of whom six patients had mutations, three patients had a mutation in exon 19, one patient in exon 20 and two patients in exon 21. Sixty-seven patients were treated with gefitinib and 35 patients were treated with erlotinib. The median duration of treatment with gefitinib was 69 days (range 14–1259 days) and with erlotinib 78 days (range 15–814 days).
Table 1: Patient and tumour characteristics (n=102)

Baseline sEGFR levels were available for all 102 patients and showed a Gaussian distribution. The mean sEGFR level at baseline was 55.9 μg/l (SD 8.9). Given the normal range provided by the manufacturer of the test (48–72 μg/l), 23% of patients had decreased sEGFR levels. Patients with a squamous cell tumour had significant lower values of sEGFR compared to patients with tumours of the undifferentiated cell type (p=0.0267); sEGFR levels of patients with adenocarcinoma were found in between. Age was the only patient's characteristic that significantly inversely correlated with sEGFR (correlation -0.31, p=0.0014). No significant associations were detected for sEGFR levels with gender, smoking status or tumour stage.
Baseline CEA values were available for 100 patients. CEA values did not follow a normal distribution. The median serum CEA value overall was 11.1 μg/l (range <1.0–2938.0 μg/l). Using the internationally accepted upper limit of normal of 6.5 μg/l for smokers and 5.0 μg/l for non-smokers, 67 patients (67%) had elevated CEA levels. Because of the skewed distribution of CEA, further analyses were performed using the logarithm of CEA (log CEA, mean 1.17, SD 0.75). Log CEA levels were significantly lower for stage III patients (p=0.01197) and for squamous cell compared to undifferentiated large cell type (p=0.0359). Values of patients with adenocarcinoma were very close to values of patients with tumours of the undifferentiated large cell type. For age, gender or level of sEGFR no association with log CEA was found.
When continuous variables (age, sEGFR and logCEA) were checked for possible non-linear associations, none were found to be significant. Consequently, the continuous variables could be included as linear continuous parameters.
The median overall survival was 5.2 months (range 0.6–52.0 months). In an univariate analysis, smoking status and sEGFR were shown to be significant prognostic factors (Table 2, p=0.001 and p=0.018, respectively). Cut-off values for sEGFR and log CEA were found at 55 μg/l and 1.1 (corresponding with CEA= 12.6 µg/l), respectively. Patients with sEGFR levels above 55 μg/l had a significantly longer overall survival (Figure 1a, log rank p=0.033), while patients with a log CEA level below 1.1 showed a trend toward longer overall survival (Figure 1b, log rank p=0.06). In a multivariate overall survival model, sEGFR and log CEA, in addition to smoking, proved to be independently associated with survival (Table 3).
Table 2: Univariate analysis in patients with advanced NSCLC before treatment with EGFR-TKIs
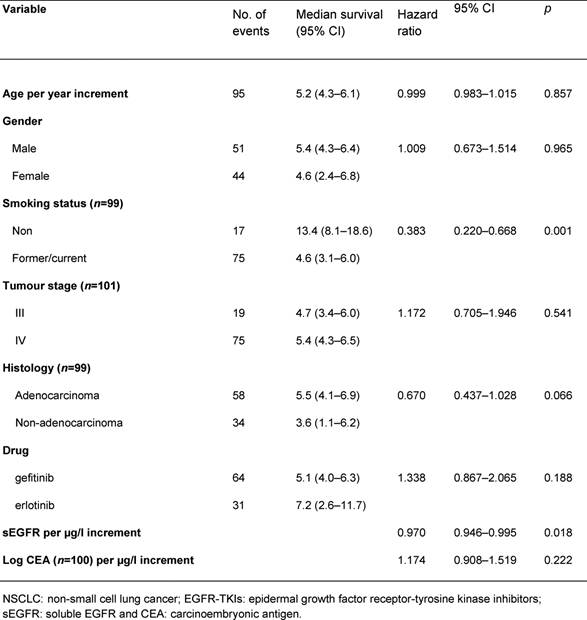
Figure 1: Overall survival by sEGFR and log
CEA
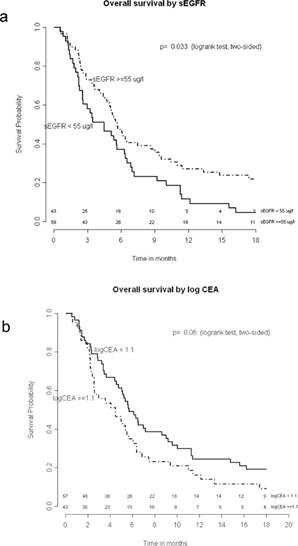
Overall survival by sEGFR (a) and log CEA (b). Numbers of patients at risk are given.
Table 3: Multivariate overall survival model in patients with advanced NSCLC before treatment with EGFR-TKIs: results of the Cox proportional hazard regression analysis
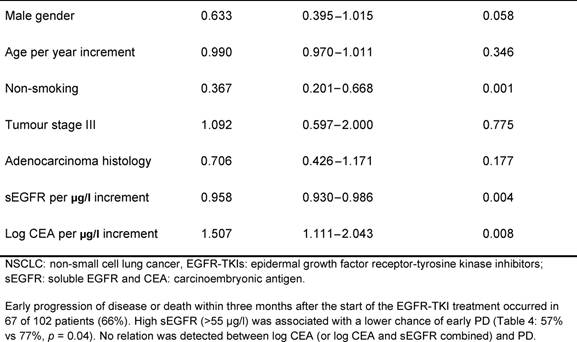
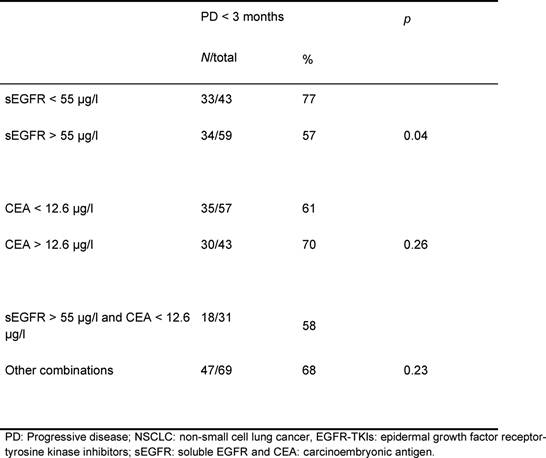
Table 4: Relation between pre-treatment sEGFR
and CEA levels and early progressive disease in patients with advanced NSCLC
after treatment with EGFR-TKIs
Discussion
In the present study, baseline sEGFR and CEA levels were measured in patients with advanced NSCLC before the treatment with erlotinib or gefitinib. Our results suggest that higher sEGFR and lower CEA levels are related to prolonged survival in patients receiving EGFR-TKI treatment, indicating that the combination of sEGFR and CEA could be valuable for the selection of patients for the EGFR-TKI treatment.
Carcinoembryonic antigen is a member of the immunoglobulin superfamily and plays a role in cell-to-cell adhesion [35]. When CEA is over-expressed on the cell surface, it is thought to play a role in tumourigenesis by disruption of cell polarity, inhibition of apoptosis (anoikis) and inhibition of cell differentiation [36–38]. The over-expression of CEA has been found to be present in many types of carcinomas [39]. In non-small cell lung cancer, an elevated serum CEA level is generally considered to be a negative prognostic factor especially for adenocarcinoma [40]. Therefore[C1], the finding of Okamoto et al. that a high CEA was predictive for good response to the EGFR-TKI treatment, independent of histology, was highly surprising [41]. They did not have an explaining mechanism of action for this phenomenon, but hypothesized that an anti-apoptotic signal of the (mutant) EGFR may somehow elevate the expression level of CEA protein. In this study, we could not confirm the results of Okamoto et al. In contrast, we found that a low CEA level was independently associated with better outcome after the treatment with EGFR-TKIs. This is in accordance with the previous findings of the negative prognostic ability of CEA [42].
Our results suggest that serum levels of CEA and sEGFR are not (directly) regulated by the same mechanism of action, since both remained significant upon multivariate analysis. Only few studies are available on sEGFR in non-small cell lung cancer, mostly concerning the comparison of sEGFR levels in healthy individuals and lung cancer patients. Two studies found that patients with NSCLC had lower baseline sEGFR levels compared to healthy controls [43,44], whereas others did not detect significant differences [45,46]. However, up till now, there are no data available on the prognostic value of serum sEGFR for NSCLC. Only one study investigated changes in the sEGFR levels during the EGFR-TKI treatment as a predictive marker for response to these inhibitors [47]. Responders showed a decrease in the sEGFR levels at time of best response compared to baseline level, whereas non-responders showed an increase. A difference of -3.6 μg/l as a cut-off was found to identify responders at time of best response. However, a meaningful cut-off level for (pre-treatment) baseline levels could not be established, and therefore, sEGFR was not considered to be a useful predictive marker. Unfortunately, in our study, serum samples drawn during treatment were not available, and we therefore could not validate these results.
The one-armed design and retrospective nature of our study prohibit clear differentiation between the prognostic and the predictive values of sEGFR and CEA. Interpretation of response data (progressive disease) remains difficult, but these data suggest at least some predictive potential for sEGFR. Higher levels of pre-treatment sEGFR in patients treated with EGFR-TKIs were associated with lower risk of progressive disease within three months.
The prognosis of advanced NSCLC after failure of second or third line treatment is generally only weeks to months. Intensive follow-up with additional imaging during this period is undesirable, and further efforts to evaluate response or progression-free survival are meaningless. The distinction between the prognostic value and the predictive value of these two markers remains important, since their potential predictive value may contribute to an adequate patient selection for expensive EGFR-TKI treatment. Therefore, validation of this potential predictive value in a prospective controlled (two-armed) study is warranted.
In conclusion, these results suggest that sEGFR and CEA are the markers of survival in patients treated with EGFR-TKIs. The potential predictive value of sEGFR needs confirmation in a prospective controlled trial.
Conflicting interests:
Dr Kappers has no conflict of interest to disclose.
Dr Vollebergh has no conflict of interest to disclose.
Mr van Tinteren has no conflict of interest to disclose.
Ms Korse has no conflict of interest to disclose.
Ms Nieuwenhuis has no conflict of interest to disclose.
Mr Bonfrer has no conflict of interest to disclose.
Dr Klomp has received an unrestricted research grant from a non-pharmaceutical family fund and from Roche (not related to this article).
Dr van Zandwijk has received honoraria from E Lilly, Merck-Serono and Roche (not related to this article).
Dr van den Heuvel has no conflict of interest to disclose.
Acknowledgement
No external sources of funding
Reference List
1. Veale D, Ashcroft T, Marsh C, Gibson GJ and Harris AL (1987) Epidermal growth factor receptors in non-small cell lung cancer Br J Cancer 55 513–6 PMID: 3038157
2. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP and Baselga J (15-6-2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol 21 2237–46 PMID: 12748244 doi:10.1200/JCO.2003.10.038
3. Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr., Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ and Kay AC (22-10-2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial JAMA 290 2149–58 PMID: 14570950 doi:10.1001/jama.290.16.2149
4. Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, Rigas J, Clark GM, Santabarbara P and Bonomi P (15-8-2004) Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer J Clin Oncol 22 3238–47 PMID: 15310767 doi:10.1200/JCO.2004.11.057
5. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP and Baselga J (15-6-2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol 21 2237–46 PMID: 12748244 doi:10.1200/JCO.2003.10.038
6. Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr., Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ and Kay AC (22-10-2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial JAMA 290 2149–58 PMID: 14570950 doi:10.1001/jama.290.16.2149
7. Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, Krug LM, Pao W, Rizvi N, Pizzo B, Tyson L, Venkatraman E, Ben Porat L, Memoli N, Zakowski M, Rusch V and Heelan RT (15-3-2004) Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer J Clin Oncol 22 1103–9 PMID: 15020612 doi:10.1200/JCO.2004.08.158
8. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P and Seymour L (14-7-2005) Erlotinib in previously treated non-small-cell lung cancer N Engl J Med 353 123–32 PMID: 16014882 doi:10.1056/NEJMoa050753
9. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V and Carroll K (29-10-2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet 366 1527–37 PMID: 16257339 doi:10.1016/S0140-6736(05)67625-8
10. Inoue A, Suzuki T, Fukuhara T, Maemondo M, Kimura Y, Morikawa N, Watanabe H, Saijo Y and Nukiwa T (20-7-2006) Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations J Clin Oncol 24 3340–6 PMID: 16785471 doi:10.1200/JCO.2005.05.4692
11. Janne PA, Engelman JA and Johnson BE (10-5-2005) Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology J Clin Oncol 23 3227–34 PMID: 15886310 doi:10.1200/JCO.2005.09.985
12. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J and Haber DA (20-5-2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350 2129–39 PMID: 15118073 doi:10.1056/NEJMoa040938
14. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE and Meyerson M (4-6-2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy Science 304 1497–500 PMID: 15118125 doi:10.1126/science.1099314
15. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M and Varmus H (7-9-2004) EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib Proc Natl Acad Sci U S A 101 13306–11 PMID: 15329413 doi:10.1073/pnas.0405220101
16. van Zandwijk N, Mathy A, Boerrigter L, Ruijter H, Tielen I, de Jong D, Baas P, Burgers S and Nederlof P (2007) EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: retro- and prospective observations in non-small-cell lung cancer Ann Oncol 18 99–103 PMID: 17060486 doi:10.1093/annonc/mdl323
17. Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I, Ludovini V, Magrini E, Gregorc V, Doglioni C, Sidoni A, Tonato M, Franklin WA, Crino L, Bunn PA, Jr. and Varella-Garcia M (4-5-2005) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer J Natl Cancer Inst 97 643–55 PMID: 15870435
18. Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, Marrano P, Cunha Santos G, Lagarde A, Richardson F, Seymour L, Whitehead M, Ding K, Pater J and Shepherd FA (14-7-2005) Erlotinib in lung cancer - molecular and clinical predictors of outcome N Engl J Med 353 133–44. PMID: 16014883 doi:10.1056/NEJMoa050736
19. Hirsch FR, Varella-Garcia M, Bunn PA, Jr., Franklin WA, Dziadziuszko R, Thatcher N, Chang A, Parikh P, Pereira JR, Ciuleanu T, von Pawel J, Watkins C, Flannery A, Ellison G, Donald E, Knight L, Parums D, Botwood N and Holloway B (1-11-2006) Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer J Clin Oncol 24 5034–42 PMID: 17075123 doi:10.1200/JCO.2006.06.3958
20. Baron AT, Lafky JM, Boardman CH, Balasubramaniam S, Suman VJ, Podratz KC and Maihle NJ (1999) Serum sErbB1 and epidermal growth factor levels as tumor biomarkers in women with stage III or IV epithelial ovarian cancer Cancer Epidemiol Biomarkers Prev 8 129–37 PMID: 10067810
21. Carney WP, Burrell M, Morris LD and Hamer PJ (2002) Normal levels of serum EGFr and decreases in several cancers Proceedings AACR Vol 43, Abstract #240
22. Choi JH, Oh JY, Ryu SK, Kim SJ, Lee NY, Kim YS, Yi SY, Shim KS and Han WS (15-5-1997) Detection of epidermal growth factor receptor in the serum of gastric carcinoma patients Cancer 79 1879–83 PMID: 9149012 doi:10.1002/(SICI)1097-0142(19970515)79:10<1879::AID-CNCR6>3.3.CO;2-R
23. Partanen R, Hemminki K, Koskinen H, Luo JC, Carney WP and Brandt-Rauf PW (1994) The detection of increased amounts of the extracellular domain of the epidermal growth factor receptor in serum during carcinogenesis in asbestosis patients J Occup Med 36 1324–8 PMID: 7884573 doi:10.1097/00043764-199412000-00013
24. Gregorc V, Ceresoli GL, Floriani I, Spreafico A, Bencardino KB, Ludovini V, Pistola L, Mihaylova Z, Tofanetti FR, Ferraldeschi M, Torri V, Cappuzzo F, Crino L, Tonato M and Villa E (15-9-2004) Effects of gefitinib on serum epidermal growth factor receptor and HER2 in patients with advanced non-small cell lung cancer Clin Cancer Res 10 6006–12 PMID: 15447984 doi:10.1158/1078-0432.CCR-03-0770
25. Gregorc V, Ceresoli GL, Floriani I, Spreafico A, Bencardino KB, Ludovini V, Pistola L, Mihaylova Z, Tofanetti FR, Ferraldeschi M, Torri V, Cappuzzo F, Crino L, Tonato M and Villa E (15-9-2004) Effects of gefitinib on serum epidermal growth factor receptor and HER2 in patients with advanced non-small cell lung cancer Clin Cancer Res 10 6006–12 PMID: 15447984 doi:10.1158/1078-0432.CCR-03-0770
26. Okamoto T, Nakamura T, Ikeda J, Maruyama R, Shoji F, Miyake T, Wataya H and Ichinose Y (2005) Serum carcinoembryonic antigen as a predictive marker for sensitivity to gefitinib in advanced non-small cell lung cancer Eur J Cancer 41 1286–90 PMID: 15939264 doi:10.1016/j.ejca.2005.03.011
27. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M and Clark GM (22-8-2005) Reporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93 387–91 PMID: 16106245 doi:10.1038/sj.bjc.6602678
28. Carney WP, Burrell M, Morris LD and Hamer PJ (2002) Normal levels of serum EGFr and decreases in several cancers Proceedings AACR Vol 43, Abstract #240
29. Molina R, Bonfrer J, Banfi G, Bugugnani MJ, Cornu F, Hannemann-Pohl K, Hubl W, Merz O, Bach M and Mack M (2000) External evaluation of LIAISON tumour marker assays on the fully automated chemiluminescent LIAISON immunoassay analyser Clin Lab 46 169–79 PMID: 10791126
30. Schoenfeld D (1982) Partial residuals for the proportional hazards regression model Biometrika 69 239–41 doi:10.1093/biomet/69.1.239
31. Therneau TM, Grambsch PM and Fleming TR (1990) Martingale-based residuals and survival models. Biometrika 77 147–60 doi:10.1093/biomet/77.1.147
32. Albain KS, Swann RS and Rusch VR et al. (2005) Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) vs CT/RT followed by surgical resection for stage IIIA (pN2) non-small cell lung cancer (NSCLC): Outcomes update of North American Intergroup trial 0139 (RTOG 9309). J Clin Oncol 23
33. van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, Tjan-Heijnen VC, Biesma B, Debruyne C, van Zandwijk N, Splinter TA and Giaccone G (21-3-2007) Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer J Natl Cancer Inst 99 442–50 PMID: 17374834
34. Van Schil P, van Meerbeeck J, Kramer G, Splinter T, Legrand C, Giaccone G, Manegold C and van Zandwijk N (2005) Morbidity and mortality in the surgery arm of EORTC 08941 trial Eur Respir J 26 192–7 PMID: 16055865 doi:10.1183/09031936.05.00127204
35. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC and Gwyther SG (2-2-2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst 92 205–16 PMID: 10655437 doi:10.1093/jnci/92.3.205
36. Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K and Stanners CP (21-4-1989) Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule Cell 57 327–34 PMID: 2702691 doi:10.1016/0092-8674(89)90970-7
37. Ordonez C, Screaton RA, Ilantzis C and Stanners CP (1-7-2000) Human carcinoembryonic antigen functions as a general inhibitor of anoikis Cancer Res 60 3419–24 PMID: 10910050
38. Screaton RA, Penn LZ and Stanners CP (1997) Carcinoembryonic antigen, a human tumor marker, cooperates with Myc and Bcl-2 in cellular transformation J Cell Biol %19 137 939–52 PMID: 9151695 doi:10.1083/jcb.137.4.939
39. Stanners CP (1998) Contributions of the human CEA family to malignant transformation in Stanners CP (ed): Cell Adhesion and Communication Mediated by the CEA Family: Basic and Clinical Perspectives. Amsterdam: Harwood Academic Publishers vol 5, pp 141–54
40. Hammarstrom S (1999) The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues Semin Cancer Biol 9 67–81 PMID: 10202129 doi:10.1006/scbi.1998.0119
41. Okamoto T, Nakamura T, Ikeda J, Maruyama R, Shoji F, Miyake T, Wataya H and Ichinose Y (2005) Serum carcinoembryonic antigen as a predictive marker for sensitivity to gefitinib in advanced non-small cell lung cancer Eur J Cancer 41 1286–90 PMID: 15939264 doi:10.1016/j.ejca.2005.03.011
42. Concannon JP, Dalbow MH, Hodgson SE, Headings JJ, Markopoulos E, Mitchell J, Cushing WJ and Liebler GA (1978) Prognostic value of preoperative carcinoembryonic antigen (CEA) plasma levels in patients with bronchogenic carcinoma Cancer 42 1477–83 PMID: 709518 doi:10.1002/1097-0142(197809)42:3+<1477::AID-CNCR2820420818>3.0.CO;2-E
43. Carney WP, Burrell M, Morris LD and Hamer PJ (2002) Normal levels of serum EGFr and decreases in several cancers Proceedings AACR Vol 43 Abstract #240
44. Lemos-Gonzalez Y, Rodriguez-Berrocal FJ, Cordero OJ, Gomez C and Paez de la Cadena M (21-5-2007) Alteration of the serum levels of the epidermal growth factor receptor and its ligands in patients with non-small cell lung cancer and head and neck carcinoma Br J Cancer 96 1569–78 PMID: 17453000 doi:10.1038/sj.bjc.6603770
45. Jacot W, Pujol JL, Boher JM and Lamy PJ (2-8-2004) Serum EGF-receptor and HER-2 extracellular domains and prognosis of non-small-cell lung cancer Br J Cancer 91 430–3 PMID: 15226769 doi:10.1038/sj.bjc.6601987
46. Schneider J, Presek P, Braun A, Bauer P, Konietzko N, Wiesner B and Woitowitz HJ (1999) p53 protein, EGF receptor, and anti-p53 antibodies in serum from patients with occupationally derived lung cancer Br J Cancer 80 1987–94 PMID: 10471051 doi:10.1038/sj.bjc.6690632
47. Gregorc V, Ceresoli GL, Floriani I, Spreafico A, Bencardino KB, Ludovini V, Pistola L, Mihaylova Z, Tofanetti FR, Ferraldeschi M, Torri V, Cappuzzo F, Crino L, Tonato M and Villa E (15-9-2004) Effects of gefitinib on serum epidermal growth factor receptor and HER2 in patients with advanced non-small cell lung cancer Clin Cancer Res 10 6006–12 PMID: 15447984 doi:10.1158/1078-0432.CCR-03-0770
[C1]Author: The author's name 'Okamoto et al.' appearing in the paragraph 'Therefore, the finding ... highly surprising [41]' does not match to the name in the corresponding Ref. [41]. Please verify.






