Clinicopathological features associated with CD44 and CD63 expression in breast cancer
Carlos A. Castaneda1,2, Miluska Castillo3, Joselyn Sanchez3, Luis Bernabe3, Katherin Tello3, Nancy Suarez3, Raul Alatrista3, Ximena Quiroz-Gil1, Alexandra Granda-Oblitas1, Javier Enciso4, Nathaly Enciso5 and Henry L Gomez6
1Faculty of Health Sciences, Universidad Cientifica del Sur, Lima 15067, Peru
2Department of Medical Oncology, Instituto Nacional de Enfermedades Neoplasicas, Lima 15038, Peru
3Department of Research, Instituto Nacional de Enfermedades Neoplasicas, Lima 15038, Peru
4Laboratorio de Células Madre, Universidad Cientifica del Sur, Lima 15067, Peru
5Direccion General de Investigacion, Desarrollo e Innovacion, Universidad Científica del Sur, Lima 15067, Peru
6Unidad de Ensayos Clinicos, Oncosalud-AUNA, Lima 15038, Peru
Abstract
Background: CD44 is a cell-surface transmembrane glycoprotein that participates in the regulation of many cellular processes, including cell division, adhesion, migration and stem-like characteristics. CD63 is involved in the exocytosis process.
Objective: To evaluate the relationship between CD44 and CD63 expression and clinicopathological features, including tumor-infiltrating lymphocytes (TILs), phosphoinositide 3-kinase (PIK3CA) mutation and survival.
Methodology: CD44 and CD63 were stained in samples from 101 breast cancer cases from Peruvian women.
Results: Median age was 52 years, most were most were grade-3 (68%), estrogen receptor (ER)+ (64%) and stage II-III (92%). Median ki67 was 30%, median stromal TIL was 30% and PIK3CA mutation was found in 49%. Longer survival was associated with earlier stages (p = 0.016), lower ki67 (p = 0.023), ER+ (p = 0.034), luminal phenotype (p = 0.029) and recurrence (p < 0.001). CD44 was classified as high cell density staining in 57% and high intensity in 55%. High CD44 density was associated with younger age (p = 0.043), triple-negative phenotype (p = 0.035) and shorter survival (p = 0.005). High CD44 expression was associated with short survival (p = 0.005). High CD63 cell density was found in 56% of cases and was associated with ER-positive (p = 0.045), low TIL levels (p = 0.007), Luminal-A (p = 0.015) and low CD44 intensity (p = 0.032).
Conclusion: CD44 expression was associated with aggressive features and low CD63 density staining.
Keywords: breast cancer, CD44, CD63, tumor-infiltrating lymphocytes
Correspondence to: Carlos A. Castaneda
Email: carloscastanedaaltamirano@yahoo.com
Published: 26/09/2024
Received: 22/01/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Breast cancer is the most common malignancy among women worldwide, including in Peru [1]. The clinicopathologic features that predict survival serve as prognostic factors. These include histologic features and the immunohistochemical status of estrogen-receptor (ER), progesterone-receptor (PgR) and HER2. Tumor-infiltrating lymphocytes (TIL) are a predictive biomarker for response to chemotherapy and immunotherapy, while phosphoinositide 3-kinase (PIK3CA) mutations predict response to targeted therapy [2].
CD44 is a cell surface transmembrane glycoprotein involved in regulating many cellular processes, including cell division, adhesion and migration by binding to its primary ligand, the hyaluronic acid [3, 4]. It promotes carcinogenesis by accelerating proliferation or suppressing apoptosis through the stimulation of Ras-Raf-Mek-Erk-cyclin D1 signaling and the PIK3CA pathway [4].
CD44 is also involved in the epithelial-mesenchymal-transition (EMT) process and in extravasation through the endothelial barrier, both of which stimulate tumor invasion and metastasis [3, 5–9]. Additionally, CD44-expressing breast cancer cells exhibit stem-cell-like characteristics and indicate a poor prognosis when detected within the bone marrow of patients with early-stage breast cancer [8–10]. Laboratory tests remarkably found that the knockdown of CD44 reduces tumor cell adhesion to endothelial cells in vitro, and decreases overall tumor burden in the brain, lung, liver and skeleton as well as increased cell survival in vivo experiments [11]. Despite this experimental data, there is no consensus on the association between CD44 expression and clinicopathological features [12–14]. Furthermore, there is no information on its expression in breast cancer series from South American countries.
Breast cancer patients have a higher number of extracellular vesicle proteins in plasma than healthy controls. The tetraspanin protein CD63 is a key factor in extracellular vesicle production and endocytosis [15]. It has been implicated in cell motility and metastatic capacity in cancer. Additionally, recent studies suggest that CD63 expression could also serve as a marker for stem cell features [16, 17]. Laboratory studies find that the exocytosis process could mediate immunosupression in the tumoral micro environment, and gene-modified mice with tetraspanin deficiency exhibit immune response alterations [18]. Unfortunatelly, few studies have evaluated the association between CD63 expression and clinical features or survival in breast cancer [15].
Additionally, different studies indicate that race acts as a prognostic factor. For instance, black women often have a worse prognosis, whereas Latina women may have a better prognosis. Various series also describe that breast tumors in Latinas have particular features, such as a younger age at diagnosis and a higher prevalence of triple-negative breast cancer (TNBC) phenotype. However, there is a lack of information about what molecular variants produce these differences [19, 20].
In this study, we evaluated the correlation between the expression of CD44, CD63 and clinicopathological features, including the level of TIL and PIK3CA mutations, as well as survival in a Peruvian breast cancer series.
Materials and methods
Patient selection
This is a retrospective study from a single institution. Patients with invasive breast cancer diagnosed between 2016 and 2018 and who have paraffin samples stored at the Instituto Nacional de Enfermedades Neoplasicas were included. The study was approved by the Ethics Committee. The clinicopathological information was obtained from the patients’ files. Additionally, an experienced pathologist reviewed the Hematoxylin Eosin (HE)-stained pathology slides to document missing histopathology data, including TILs according to the international TILs working group recommendation (JS) [21].
Evaluation of CD44 and CD68 staining in paraffin samples
Tissue microarrays (TMAs) were constructed from tumor areas obtained from paraffin blocks with 6.0 mm diameter cores. Serial 4 μm sections were prepared and used for immunohistochemical (IHC) staining. Sections of paraffin samples were rehydrated in phosphate-buffered saline (PBS) and immersed in 0.1% trypsin solution in PBS at 37°C for 5 to 10 minutes or microwaved for 5 minutes for 4 (total, 20 minutes) in buffer to recover the antigen. Sections were treated for 45 minutes with 10% normal goat serum or normal horse serum in PBS. The primary anti-human antibodies used for IHC (following manufacturer’s instructions) were rabbit anti-CD44 monoclonal antibody (clone SP37, MAD-000537QD, ready-to-use, Vitro Master Diagnostica, Granada, Spain) and CD63 (clone NKI/C3, MAD-000543QD, ready-to-use, Vitro Master Diagnostica, Granada, España). Sections were incubated in alkaline phosphatase-streptavidin (Vector Laboratories, Burlingame, CA; 1:1,000 dilution) for 30 minutes at room temperature, and reacted with Fast-Red Substrate System (Dakopatts) and Dako® Fuchsin + Substrate- Chromogen. Background staining was performed with Mayer’s hematoxylin solution, and then sections were dehydrated through rising alcohols to xylene and mounted on slides.
The stained slides were read by a pathologist (JS) and categorized into three categories, status, density and intensity. Density was classified as high when brown staining was found in at least 10% (median value and previously described cutoff [22, 23]) of cancer cells with the anti-CD44 reactive, and when at least 80% (median value) of cancer cells were stained with anti-CD63 slides. The intensity of CD44 and CD63 was high when the darker degree of staining was observed in the nucleus and membrane locations, respectively (Figure 1).
Detection of PIK3CA mutation
DNA was extracted from paraffin-embedded tumor samples using the QIAamp DNA FFPE tissue kit (Qiagen; Hilden, Germany) [24]. TaqMan-based real-time PCR analysis was carried out using the LightCycler® 96 PCR System (Roche Applied Science, Mannheim, Germany) to detect the three ‘hot spot’ PIK3CA mutations (H1047R, E545K and E542K). Custom TaqMan primers and probes were designed for PIK3CA mutations (PI3KCA 760: c.1624 G (VIC) >A (FAM), PI3KCA 763: c.1633 G (VIC) >A (FAM), PI3KCA 775: c.3140 A (VIC) >G (FAM), Scientific ThermoFisher). The thermal cycler protocol was: 10 minutes at 96°C, 39 cycles at 60°C for 2 minutes, 98°C for 30 seconds and 60°C for 1 minutes. Each mutation was analysed in a single assay for all samples. The mutational threshold was determined to be 1% after measuring the WT HDx FFPE reference standards (Horizon Diagnostics, Cambridge, United Kingdom). On the other hand, the threshold for reagents that induced false positives was assumed to be 0.5% of the mutation frequency.
Statistical analysis
Relationships between expression status and clinicopathological features were detected using Chi-square or Fisher’s exact tests. Median follow-up was 5.48 years, and survival rates were calculated from diagnosis to recurrence or last follow-up date/death, and estimated using the Kaplan-Meier method. p values less than 0.05 were considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows version 26.0 (SPSS Inc., Chicago, IL, USA).
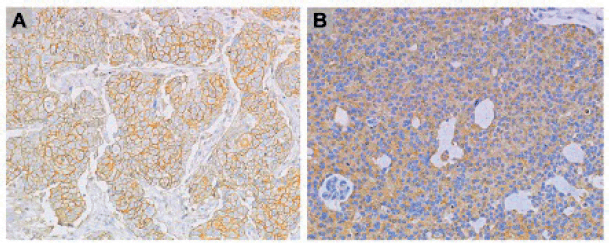
Figure 1. (A): CD44 and (B): CD63 IHC staining at 20× magnification.
Results
Clinical and pathological features
It included 101 cases of breast cancer in Peruvian women and the median age was 52 years. Most had NST histology (94%), grade 3 (68%), ER-positive (64.4%), luminal-B phenotype (45.5%), TNBC (21.8%) and stage II–III (92%). Carcinoma in-situ was found in 65 cases (65%). The median ki67 was 30%, the median stromal TIL was 30%, and the PIK3CA mutation was found in 49% (n = 49). Only one mutation was found in 27 cases (14 cases with H1047R, 8 with E545K and 5 with E542K). Two mutations were found in 15 (9 cases with H1047R and E545K, 1 with H1047R and E542K and 5 with E542K and E545K). The three mutations were found in seven cases.
Recurrence was found in 21 cases and death in 26. Longer overall survival was associated with earlier stages (p = 0.016), lower ki67 (p = 0.023), ER+ (p = 0.034), luminal phenotype (p = 0.029) and the absence of recurrence (p < 0.001) (Table 1, Figure 2).
Table 1. Clinicopathological features and outcomes.
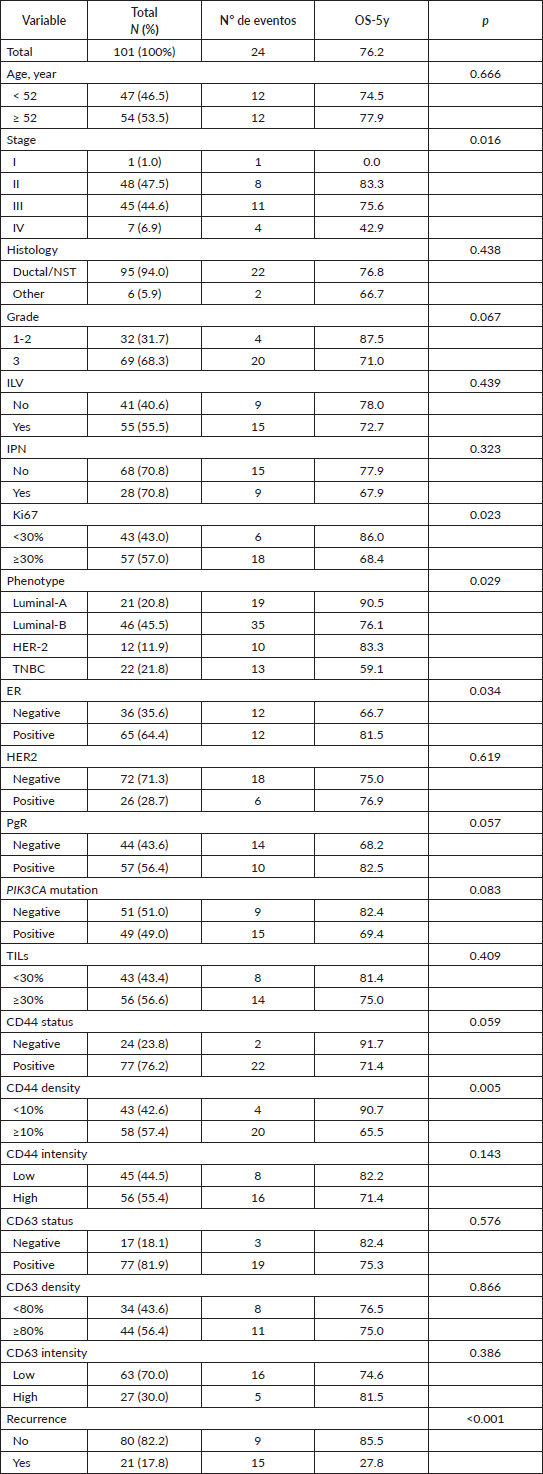
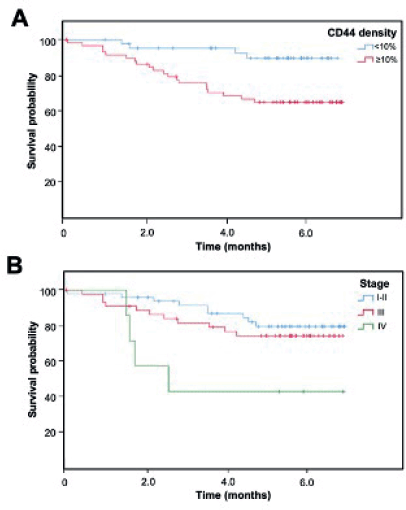
Figure 2. Kaplan-Meier overall survival curve according to (A) CD44 density and (B) clinical stage.
Relationship between CD44 and tumor features
CD44 was classified as positive status in 77 (76.2%), high density (larger or equal than 10%) in 58 (57.4%) and high intensity in 56 (55.4%) (Table 1). The high density of CD44 was associated with younger age (p = 0.043), triple-negative (p = 0.035), shorter overall survival (p = 0.005) and a trend for RE-negative status (p = 0.162). CD44 status was not associated with the presence of in-situ carcinoma, TIL levels, PIK3CA mutations (p = 0.898) nor any of the three specific mutations (p = 0.144–0.862) (Table 2, Figure 2).
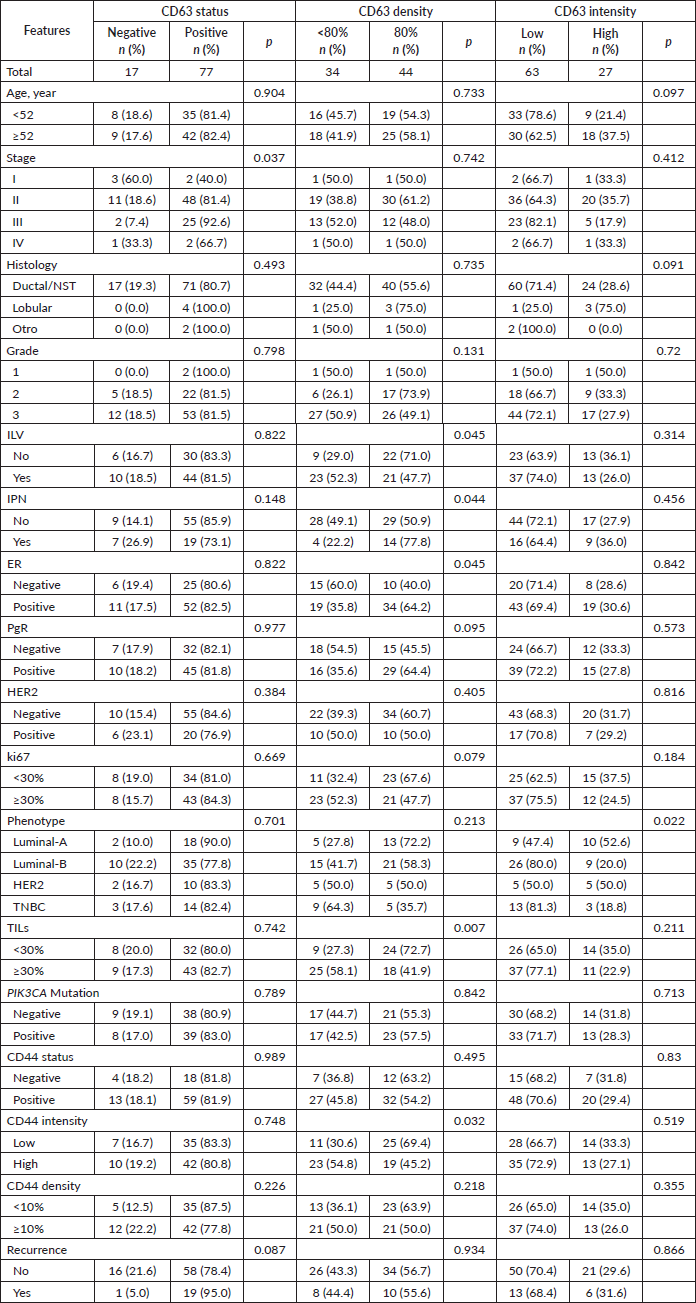
Relationship between CD63 and tumor features
CD63 was classified as positive status in 77 (81.9%), high density (larger or equal to the median value, 80%) in 44 (56.4%) and high intensity in 27 (30%). High density was associated with the absence of both lymphovascular invasion (p = 0.045) and perineural invasion (p = 0.044), as well as with ER-positive (p = 0.045) and low TIL levels (p = 0.007). High CD63 intensity was associated with Luminal-A (52.6% vs. 23.9%, p = 0.015) and with non-Luminal-B (20% vs. 40%, p = 0.038) phenotypes.
High CD63 density was associated with low CD44 intensity (p = 0.032).
CD63 expression was not associated with the presence of in-situ carcinoma, PIK3CA mutations (p = 0.789) nor any of the three mutations (p = 0.308–0.887) (Table 3).
Table 2. Relationship between CD44 and clinicopathological features.
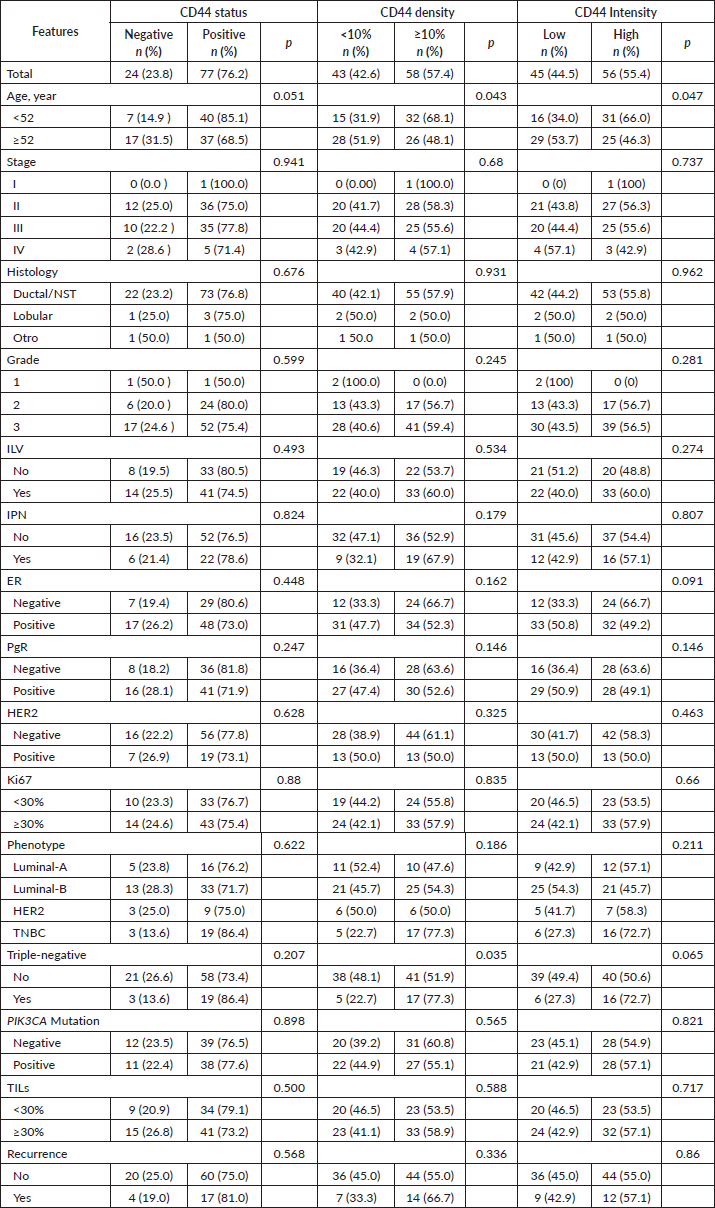
Table 3. Relationship between CD63 and clinicopathological features.
Discussion
Our analysis showed that high CD44 protein expression was associated with young age, TNBC phenotype and shorter survival. As previously mentioned, laboratory experiments indicate that CD44 is a CSC marker, an important up-regulator of the EMT process, and promotes metastasis and chemotherapy resistance [3, 25]. Other tumor sample analysis have also described this aggressive feature of CD44 staining. Gudadze et al. found that CD44 protein was associated with ER-negative status, luminal-B and TNBC phenotypes as well as larger tumor size and node involvement in their series of 393 breast cancer series [26]. Similarly, McFarlane et al., described that CD44 expression was associated with high grade (p = 0.046), ER-negative (p = 0.001), PgR-negative status (p = 0.029) and higher recurrence rates in their 448 breast cancer cases [11]. Similarly, other groups have also found an association between CD44 and the TNBC phenotype [27, 28].
Our finding of an association between CD44-positive and shorter survival has also been described by other groups. McFarlane et al. found that CD44 expression was correlated with increased distant recurrence and reduced disease-free survival in patients with lymph node-positive or large tumors [11]. Finally, a systematic review including nine studies found that CD44-positive was associated with inferior survival, larger tumor size, higher grade and node involvement in TNBC [29].
Our analysis did not find that CD44 expression modulates TIL levels, despite the fact that CD44 expression has recently been linked with the increased expression of PD-L1 in TNBC cells [30]. We also did not find that co-presence of in-situ cancer cells would be associated with overexpression of CD44, as previously published [31].
Finally, previous studies have described that populations with extensive African ancestry have high rates of tumors with stem cell features as well as TNBC rates [32, 33]. Therefore, our findings could also suggest that the high prevalence of TNBC in our Latinas series could be associated with high rates of stem cell tumor features in this population [20, 34].
On the other side, our finding of an association between the expression level of the vesicle protein CD63 and all favorable prognostic features—ER-positive, Luminal-A phenotype, low TIL levels and low CD44 (a marker of stem-cell features)—has not been previously described in breast cancer.
Other series have also described that CD63 levels are inversely related to cancer invasion and metastases [35]. Similarly, Huang et al [36] describe that CD63 expression inhibits proliferation, migration, EMT and stemness in head and neck cancer. The relationship between tetraspanins and stem cell features has been previously described, as vesicles are vehicles for transmitting self-renewing and multilineage differentiating capabilities among cells [37]. Our finding that Luminal-A and ER-positive tumors have higher CD63 expression could indicate that vesicles could also transmit information from this group of cancer cells.
Our results differ from other publications that indicate CD63 levels and other tetraspanins are associated with aggressive features and poor prognosis in various malignancies [38–40] including breast cancer [41, 42]. This opposite finding could be related to the evaluation technique.
Our results found some associations between features of breast cancer and biomarkers related to stem cell features and cell vesicle production. This information has been generated in women from South America, and recent studies in precision medicine indicate that genomic variants could predispose to particular tumor features in the Peruvian population [19, 20].
More South American populations need to be included in research evaluating biomarkers to increase the knowledge of this pathology.
Conclusion
High levels of CD44 are associated with shorter survival and TNBC phenotype. CD63 distribution is inversely associated with CD44 distribution. High CD63 cell density is also associated with ER-positive, low TIL levels and Luminal-A phenotype.
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by CONCYTEC (contract 007-2016-FONDECYT) and Universidad Científica del Sur.
Institutional review
The conduct of this survey was approved by The Institutional Review Board of INEN (#INEN-16-30). Since the study was based on a secondary source and there was no contact with the patients, no informed consent was applied; however, the identity and personal data of patients’ medical records were protected at all times.
References
1. Instituto Nacional de Enfermedades Neoplasicas (2021) Metropolitan lima cancer registry. Incidence and mortality 2013–2015 ed Payet E (Lima: INEN) pp 27–28
2. Schmidt-Straßburger U (2022). Molecular and cellular analyses of breast cancers in real life Improving Oncology Worldwide vol 1, 1st edn, ed U Schmidt-Straßburger (Ulm: University of Ulm) https://doi.org/10.1007/978-3-030-96053-7
3. Xu H, Tian Y, and Yuan X, et al (2015) The role of CD44 in epithelial-mesenchymal transition and cancer development OncoTargets Ther 8 3783–3792 https://doi.org/10.2147/OTT.S95470
4. Gotte M and Yip GW (2006) Heparanase, hyaluronan, and CD44 in cancers: a breast carcinoma perspective Cancer Res 66(21) 10233–10237 https://doi.org/10.1158/0008-5472.CAN-06-1464 PMID: 17079438
5. Erb U, Megaptche AP, and Gu X, et al (2014) CD44 standard and CD44v10 isoform expression on leukemia cells distinctly influences niche embedding of hematopoietic stem cells J Hematol OncolJ Hematol Oncol 7(1) 1–19 https://doi.org/10.1186/1756-8722-7-29
6. Mastro AM, Gay CV, and Welch DR (2003) The skeleton as a unique environment for breast cancer cells Clin Exp Metastasis 20(3) 275–284 https://doi.org/10.1023/A:1022995403081 PMID: 12741685
7. Orr FW, Wang HH, and Lafrenie RM, et al (2000) Interactions between cancer cells and the endothelium in metastasis J Pathol 190(3) 310–329 https://doi.org/10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH520>3.0.CO;2-P PMID: 10685065
8. Hill A, McFarlane S, and Mulligan K, et al (2006) Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells Oncogene 25(45) 6079–6091 https://doi.org/10.1038/sj.onc.1209628 PMID: 16652145
9. Draffin JE, McFarlane S, and Hill A, et al (2004) CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells Cancer Res 64(16) 5702–5711 https://doi.org/10.1158/0008-5472.CAN-04-0389 PMID: 15313910
10. Balic M, Lin H, and Young L, et al (2006) Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype Clin Cancer Res 12(19) 5615–5621 https://doi.org/10.1158/1078-0432.CCR-06-0169 PMID: 17020963
11. McFarlane S, Coulter JA, and Tibbits P, et al (2015) CD44 increases the efficiency of distant metastasis of breast cancer Oncotarget 6(13) 11465 https://doi.org/10.18632/oncotarget.3410 PMID: 25888636 PMCID: 4484469
12. Diaz LK, Zhou X, and Wright ET, et al (2005) CD44 expression is associated with increased survival in node-negative invasive breast carcinoma Clin Cancer Res 11(9) 3309–3314 https://doi.org/10.1158/1078-0432.CCR-04-2184 PMID: 15867228
13. Dan T, Hewitt S, and Ohri N, et al (2014) CD44 is prognostic for overall survival in the NCI randomized trial on breast conservation with 25 year follow-up Breast Cancer Res Treat 143(1) 11–18 https://doi.org/10.1007/s10549-013-2758-9
14. Xu H, Tian Y, and Yuan X, et al (2016) Enrichment of CD44 in basal-type breast cancer correlates with EMT, cancer stem cell gene profile, and prognosis OncoTargets Ther 9 431–444 https://doi.org/10.2147/OTT.S97192
15. Koh HM, Jang BG, and Kim DC (2020) Prognostic value of CD63 expression in solid tumors: a meta-analysis of the literature In Vivo 34(5) 2209–2215 https://doi.org/10.21873/invivo.12031 PMID: 32871743 PMCID: 7652479
16. Marni R, Chakraborty A, and Malla R (2022) Oncogenic tetraspanins: Implications for metastasis, drug resistance, cancer stem cell maintenance and diagnosis of leading cancers in females Gene Rep 27(22) 101548 https://doi.org/10.1016/j.genrep.2022.101548
17. D’Angelo RC, Liu XW, and Najy AJ, et al (2014) TIMP-1 via TWIST1 induces EMT phenotypes in human breast epithelial cells Mol Cancer Res 12(9) 1324–1333 https://doi.org/10.1158/1541-7786.MCR-14-0105 PMCID: 4171133
18. Schaper F and Van Spriel AB (2018) Antitumor immunity is controlled by tetraspanin proteins Front Immunol 9 e1185 https://doi.org/10.3389/fimmu.2018.01185
19. Zavala VA, Casavilca-Zambrano S, and Navarro-Vasquez J, et al (2023) Breast cancer subtype and clinical characteristics in women from Peru Front Oncol 13 e938042 https://doi.org/10.3389/fonc.2023.938042
20. Castaneda CA, Castillo M, and Villarreal-Garza C, et al (2018) Genetics, tumor features and treatment response of breast cancer in Latinas Breast Cancer Manag 7(1) BMT01 https://doi.org/10.2217/bmt-2017-0013
21. Salgado R, Denkert C, and Demaria S, et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014 Ann Oncol 26(2) 259–271 https://doi.org/10.1093/annonc/mdu450
22. Roosta Y, Sanaat Z, and Nikanfar AR, et al (2020) Predictive value of CD44 for prognosis in patients with breast cancer Asian Pac J Cancer Prev 21(9) 2561–2567 https://doi.org/10.31557/APJCP.2020.21.9.2561 PMID: 32986353 PMCID: 7779424
23. Ricardo S, Vieira AF, and Gerhard R, et al (2011) Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype J Clin Pathol 64(11) 937–946 https://doi.org/10.1136/jcp.2011.090456 PMID: 21680574
24. Castaneda CA, Castillo M, and Bernabe LA, et al (2021) A biomarker study in Peruvian males with breast cancer World J Clin Oncol 12(10) 926–934 https://doi.org/10.5306/wjco.v12.i10.926 PMID: 34733614 PMCID: 8546657
25. Uchino M, Kojima H, and Wada K, et al (2010) Nuclear β-catenin and CD44 upregulation characterize invasive cell populations in non-aggressive MCF-7 breast cancer cells BMC Cancer 10(1) 1–15 https://doi.org/10.1186/1471-2407-10-414
26. Gudadze M, Kankava K, and Mariamidze A, et al (2013) Distribution of CD44/CD24 positive cells in ductal invasive carcinoma of breast of different grade and molecular subtype Georgian Med News 2013(222) 50–57
27. Idowu MO, Kmieciak M, and Dumur C, et al (2012) CD44+/CD24−/low cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome Hum Pathol 43(3) 364–373 https://doi.org/10.1016/j.humpath.2011.05.005
28. Honeth G, Bendahl PO, and Ringner M, et al (2008) The CD44+/CD24-phenotype is enriched in basal-like breast tumors Breast Cancer Res 10(3) 1–12 https://doi.org/10.1186/bcr2108
29. Abdoli Shadbad M, Hosseinkhani N, and Asadzadeh Z, et al (2021) A systematic review to clarify the prognostic values of CD44 and CD44+ CD24-phenotype in triple-negative breast cancer patients: lessons learned and the road ahead Front Oncol 11 689839 https://doi.org/10.3389/fonc.2021.689839
30. Kong T, Ahn R, and Yang K, et al (2020) CD44 promotes PD-L1 expression and its tumor-intrinsic function in breast and lung cancers Cancer Res 80(3) 444–457 https://doi.org/10.1158/0008-5472.CAN-19-1108
31. Makki J, Myint O, and Wynn AA, et al (2015). Expression distribution of cancer stem cells, epithelial to mesenchymal transition, and telomerase activity in breast cancer and their association with clinicopathologic characteristics Clin Med Insights Pathol 8 1–16 https://doi.org/10.4137/CPath.S19615 PMID: 25624778 PMCID: 4287054
32. Wu Y, Sarkissyan M, and Elshimali Y, et al (2013) Triple negative breast tumors in African-American and Hispanic/Latina women are high in CD44+, low in CD24+, and have loss of PTEN PloS One 8(10) e78259 https://doi.org/10.1371/journal.pone.0078259 PMID: 24167614 PMCID: 3805609
33. Nakshatri H, Anjanappa M, and Bhat-Nakshatri P (2015) Ethnicity-dependent and-independent heterogeneity in healthy normal breast hierarchy impacts tumor characterization Sci Rep 5(1) 13526 https://doi.org/10.1038/srep13526
34. Tiezzi DG, Valejo FAM, and Marana HRC, et al (2012) CD44+/CD24− cells and lymph node metastasis in stage I and II invasive ductal carcinoma of the breast Med Oncol 29(3) 1479–1485 https://doi.org/10.1007/s12032-011-0014-x
35. Zhou Z, Yang Z, and Zhou L, et al (2023) The versatile roles of testrapanins in cancer from intracellular signaling to cell–cell communication: cell membrane proteins without ligands Cell Biosci 13(1) 1–18 https://doi.org/10.1186/s13578-023-00995-8
36. Huang Q, Shen YJ, and Hsueh CY, et al (2023) Tetraspanin CD63 reduces the progression and metastasis of head and neck squamous cell carcinoma via KRT1-mediated cell cycle arrest Heliyon 9(7) e17711 https://doi.org/10.1016/j.heliyon.2023.e17711 PMID: 37455999 PMCID: 10344705
37. Moeinzadeh L, Razeghian-Jahromi I, and Zarei-Behjani Z, et al (2022) Composition, biogenesis, and role of exosomes in tumor development Stem Cells Int 2022(12) e8392509 https://doi.org/10.1155/2022/8392509
38. Kaprio T, Hagström J, and Andersson LC, et al (2020) Tetraspanin CD63 independently predicts poor prognosis in colorectal cancer Histol Histopathol 5(8) 887–892 https://doi.org/10.14670/HH-18-209
39. Miki Y, Yashiro M, and Okuno T, et al (2018) Clinico-pathological significance of exosome marker CD63 expression on cancer cells and stromal cells in gastric cancer PLoS One 13(9) e0202956 https://doi.org/10.1371/journal.pone.0202956 PMID: 30222750 PMCID: 6141093
40. Lewitowicz P, Matykiewicz J, and Koziel D, et al (2016) CD63 and GLUT-1 overexpression could predict a poor clinical outcome in GIST: a study of 54 cases with follow-up Gastroenterol Res Pract 2016(8) e6478374 https://doi.org/10.1155/2016/6478374
41. Yuan C, Hongxia W, and Ying Z, et al (2023) Expression of tetraspanin 1 in breast cancer and its mechanism in promoting the progression of breast cancer J Shanghai Jiao Tong Univ Med Sci 43(3) 293–300
42. Tominaga N, Hagiwara K, and Kosaka N, et al (2014) RPN2-mediated glycosylation of tetraspanin CD63 regulates breast cancer cell malignancy Mol Cancer 13(134) 1–11 https://doi.org/10.1186/1476-4598-13-134






