Tyrosine crystal deposition in pleomorphic adenoma: a rare presentation in a male smoker with long-term follow-up
Sandhya Tamgadge1, Gokul Venkateshwar2, Treville Pereira1, Avinash Tamgadge1 and Simran Pethani1
1Department of Oral & Maxillofacial Pathology and Microbiology, D.Y. Patil University School of Dentistry, Sector 7, Nerul, Navi Mumbai 400706, Maharashtra, India
2Department of Oral & Maxillofacial Surgery, D.Y. Patil University School of Dentistry, Sector 7, Nerul, Navi Mumbai 400706, Maharashtra, India
Abstract
This case report presents the diagnosis and management of a pleomorphic adenoma in a 55-year-old male smoker with a habit of smoking tobacco for 6 years. The patient presented with a chief complaint of swelling in the posterior palatal region. Clinical examination revealed a well-circumscribed, non-tender, firm swelling in the palatal region. An incisional biopsy followed by surgical excision was performed, and histopathological evaluation confirmed the diagnosis of pleomorphic adenoma. Tyrosine crystals were observed within the tumour stroma, providing additional diagnostic insight. A 10-year post-operative follow-up revealed no recurrence of the lesion.
Keywords: adenoma, pleomorphic, tobacco smoking, tyrosine, crystals, minor salivary gland
Correspondence to: Sandhya Tamgadge
Email: sandhya.tamgadge@gmail.com
Published: 23/09/2024
Received: 29/05/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Pleomorphic adenoma is the most common benign tumour of the salivary glands, characterized by its morphological complexity and diverse histological patterns. This neoplasm derives its name from the architectural pleomorphism seen microscopically, featuring a mix of epithelial and myoepithelial cells arranged in various structural patterns within a mesenchymal-like background [1]. The tumour’s complexity stems from its biphasic nature, demonstrating both epithelial and stromal components, which can vary greatly in proportion and appearance [2]. This case report illustrates this complexity through the observation of multiple cell types (including clear cells, plasmacytoid cells and spindle-shaped cells), the presence of rare tyrosine crystals and areas of osteoid tissue formation within the tumour. Furthermore, the long-term follow-up and discussion of potential etiological factors, such as smoking, contribute to our understanding of the tumour’s biological behavior and risk factors, underscoring the multifaceted nature of pleomorphic adenoma [3]. In this case report, we present a distinctive clinical scenario of a 55-year-old male smoker with a 6-year history of tobacco use. The patient presented with a swelling in the posterior palate, which upon clinical evaluation, was found to be well-defined and non-tender. An incisional biopsy followed by surgical excision confirmed the diagnosis of pleomorphic adenoma. Notably, histopathological examination revealed the presence of tyrosine crystals within the tumour stroma, a rare but significant finding [4]. This observation underscores the importance of meticulous histological analysis in elucidating the nature of salivary gland neoplasms [2, 5]. Furthermore, the 10-year post-operative follow-up, characterized by the absence of recurrence, underscores the efficacy of the chosen management strategy in achieving long-term clinical outcomes [6]. Through this case report, we aim to contribute to the existing literature on salivary gland tumours, emphasizing the relevance of comprehensive diagnostic approaches and tailored therapeutics.
Case report
A 55-year-old male presented with a swelling in the posterior palatal region, with no relevant medical history aside from a 6-year history of smoking tobacco, and consuming six packets of beedis per day. Clinical examination revealed a well-circumscribed, non-tender, firm swelling extending from tooth 13 to beyond tooth 18, though no swelling was observed extraorally. An incisional biopsy and histopathological evaluation confirmed the diagnosis of pleomorphic adenoma, with the notable finding of tyrosine crystals within the tumour stroma (Figure 1).
Surgical management was performed under general anesthesia using a transoral approach for optimal access to the lesion in the right palate. A full-thickness mucoperiosteal flap was raised from the central incisor to the maxillary right first molar (tooth 18), exposing the underlying hard palate. The lesion was carefully excised in its entirety, ensuring clear margins and minimizing trauma to adjacent tissues. Following the excision, a peripheral ostectomy of the hard palate was performed to remove any potentially involved bone.
The excised lesion was preserved for histopathological evaluation, and meticulous hemostasis was achieved before the mucoperiosteal flap was repositioned and sutured for proper healing. The patient was provided with detailed postoperative care instructions and scheduled for regular follow-up appointments to monitor healing and evaluate the histopathological findings (Figure 2).
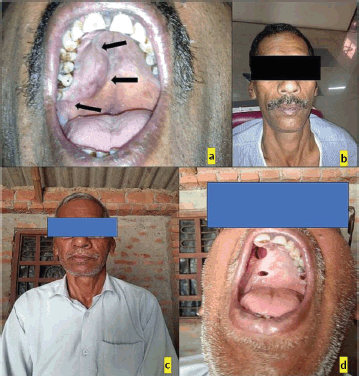
Figure 1. (a): Intraoral photograph, (b and c): Extraoral photograph, (d): Follow-up after 10 years.
Histopathological post-surgery evaluation confirmed the diagnosis of pleomorphic adenoma. The specimen showed keratinized epithelium with lesional sheets of cells encased within a capsule and islands of minor salivary gland acini in the surrounding tissue. The lesional cells exhibited a variety of morphological features, including clear, plasmacytoid and spindle-shaped cells, indicative of different myoepithelial cell types. Notably, areas of osteoid tissue added to the histological complexity of the lesion. The presence of tyrosine crystals, dispersed throughout the lesional tissues and capsule in intricate floret-like arrangements, was a striking finding. Additionally, some areas contained luminal structures lined by flat cells resembling endothelial cells (Figures 3 and 4).
A 10-year post-operative follow-up revealed no recurrence of the lesion, indicating successful management. The patient had oroantral fistula for which he has been using a prosthesis.
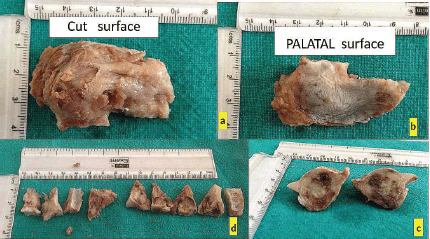
Figure 2. (a–d): Excisional biopsy specimen.
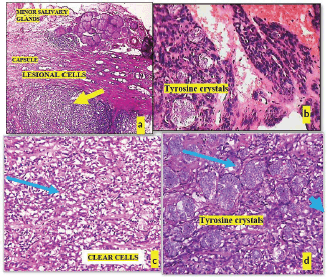
Figure 3. (a): Microphotograph shows lesional tissue (yellow arrow) surrounded by capsule, (b): Tryrosine crystals within lesional tissue, (c): Tumour composed of clear cells (blue arrow) and (d): Plenty of tyrosine crystals are seen (blue arrow).
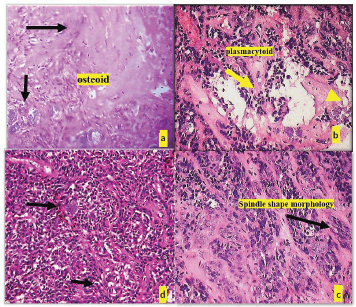
Figure 4. (a): Deposition of osteoid tissue (black arrow) and (b): Cells with plamacytoid morphology (yellow arrow), (c): Clear cell morphology of lesional cells (black arrow), (d): Cells with spindle cells morphology (black arrow).
Discussion
In this case, report of a 55-year-old male smoker with a 6-year history of tobacco use, the presence of tyrosine crystals within the tumour stroma of the pleomorphic adenoma is a distinctive finding that aided the histological diagnosis, consistent with the observations of Harris and Shipkey [7–9] in 1986.
Myoepithelial cells, with their spindle-shaped morphology and contractile properties, play a key role in the pathogenesis of the lesion. They are integral to salivary gland architecture, contributing to tissue integrity and regulating glandular secretion [10].
The presence of tyrosine-rich crystals in pleomorphic salivary adenoma (PSA) has been a subject of interest in the literature for several decades. Friedmann et al [11] were among the first to report these crystals in salivary gland tumours, highlighting their diagnostic significance. Subsequently, Valente et al [12] provided a more detailed characterization of these crystalloids using scanning electron microscopy and biochemical analysis, confirming their tyrosine-rich composition.
Margaritescu et al [6] conducted a comprehensive study on the tumoural stroma in PSA, including crystalline structures. Their findings enhanced the understanding of the tumour’s microenvironment and the potential role of these crystals in tumour biology.
In the etiopathogenesis of pleomorphic adenoma, it is proposed that genetic alterations or aberrant signalling pathways such as PLAG1 [13, 14] lead to uncontrolled proliferation and abnormal differentiation of myoepithelial cells, resulting in the formation of the characteristic mixed tumour composed of epithelial and myoepithelial components as discussed by various authors [10, 14, 15].
The clinical presentation showed a well-circumscribed, non-tender, firm swelling in the palatal region, extending from tooth 13 to beyond tooth 18. Histopathological examination revealed abundant tyrosine crystals in floret-like arrangements within the lesion, consistent with recent observations by Phulware et al [4] in parotid gland pleomorphic adenomas, underscoring the morphological diversity of these structures.
While the exact mechanism behind tyrosine crystal formation remains unclear, it has been postulated to be associated with altered metabolic processes or tissue breakdown as suggested by Eveson and Cawson [16]. The proposed origin involves metabolic dysregulation of tyrosine, a non-essential amino acid, within the tumour microenvironment, leading to its accumulation and crystallization as mentioned by Margaritescu et al [6], Phulware et al [4]. A detailed structure of tyrosine crystal has been described in the literature by Mostand and Romming [17].
The patient’s smoking history raises questions about smoking’s role in the pathogenesis of salivary gland tumours, as highlighted by several epidemiological studies, including one by Mashberg et al [18], which have reported an increased risk of salivary gland neoplasms among smokers, although the underlying mechanisms are not well understood.
The presence of tyrosine-rich crystals is not limited to pleomorphic adenoma. Gould et al [9] reported these crystalloids in adenoid cystic carcinoma, while Skálová et al [19] observed them in oncocytic cystadenoma of the parotid gland. Bellizzi and Mills [20] described collagenous crystalloids in myoepithelial carcinoma, suggesting a broader significance of crystalline structures in salivary gland pathology.
Tyrosine-rich crystalloids are primarily linked to salivary gland tumours but have also been found in various glandular and non-glandular tumours, including malignant melanoma, chondroid syringoma, vocal cord tumours, nasolabial cysts and salivary gland cysts [21–25]. For instance, Bellizzi and Mills [20] reported collagenous crystalloids in myoepithelial carcinoma, and Reinertsen et al [26] observed these structures in a meningioma case. Additionally, Amanda et al [22] identified tyrosine crystals in the cutaneous pathology of chondroid syringoma (mixed tumour). It has also been observed in nasolabial cysts in cytology specimen [27]. These observations emphasizing the importance of careful examination and interpretation.
Although the presence of tyrosine crystals in pleomorphic adenomas is linked to specific clinicopathological features, their prognostic implications are still under investigation. Phulware et al [4] have explored the potential association between tyrosine crystal formation and favourable outcomes in salivary gland tumours, but further investigations are needed to validate their prognostic utility and elucidate their role in tumour biology.
In our case report, the presence of tyrosine crystals with rare floret-like morphology provided valuable diagnostic insight into the pleomorphic adenoma of a 55-year-old male smoker with palatal swelling. Surgical excision was performed via a transoral approach with a full-thickness mucoperiosteal flap and peripheral ostectomy of the hard palate. A 10-year postoperative follow-up showed no recurrence, supporting the effectiveness of this management approach. This case emphasizes the importance of comprehensive histopathological evaluation and encourages further exploration of the diagnostic and prognostic significance of these crystalline structures in salivary gland pathology [28].
In the context of cytopathology, Carson et al [25] and Lemos et al [24] reported tyrosine crystals in fine-needle aspirates of benign parotid gland cysts and salivary gland adenomas, respectively. These findings underscore the potential diagnostic value of identifying these crystals in cytological specimens.
Conclusion
In conclusion, the literature consistently reports the presence of tyrosine-rich crystals in PSA and other salivary gland tumours. Their morphological diversity, as exemplified by our case and recent reports of floret-like structures, adds to the complex histopathological picture of these neoplasms. While their exact biological role remains to be fully elucidated, the consistent observation of these crystals across multiple studies underscores their potential diagnostic and biological significance in salivary gland pathology.
Acknowledgments
The authors would like to acknowledge lab technician Mrs Rajshree Dahale for meticulously preserving registry data.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
1. Neville BW, Damn DD, and Allen CM, et al (2002) Oral and Maxillofacial Pathology (Philadelphia: W. B. Saunders) pp 513–519
2. Sapp JP, Wysocki GP, and Eversole LR (2004) Contemporary Oral and Maxillofacial Pathology 2nd edn (St. Louis: Mosby)
3. Shafer W and Hine L Shafer’s Textbook of Oral Pathology 9th edn (Amsterdam: Elsevier)
4. Phulware RH, Dutta R, and Subramanian P, et al (2022) Rare floret like tyrosine crystals in pleomorphic adenomas of parotid gland Indian J Otolaryngol Head Neck Surg [Internet] 74(s2) 1797–1799 [https://doi.org/10.1007/s12070-020-01807-y] PMID: 36452597 PMCID: 9702217
5. Renehan AG, Gleave EN, and Slevin NJ, et al (1999) Clinico-pathological and treatment-related factors influencing survival in parotid cancer Br J Cancer 80(8) 1296–1300 https://doi.org/10.1038/sj.bjc.6990501 PMID: 10376987 PMCID: 2362357
6. Margaritescu C, Raica M, and Simionescu C, et al (2005) Tumoral stroma of salivary pleomorphic adenoma – histopathological, histochemical and immunohistochemical study Rom J Morphol Embryol 46(3) 211–223
7. Harris BR and Shipkey F (1986) Tyrosine-rich crystalloids in neoplasms and tissues of the head and neck Arch Pathol Lab Med 118(10) 709–712
8. Torous VF, Dodd LG, and McIntire PJ, et al (2022) Crystals and crystalloids in cytopathology: incidence and importance Cancer Cytopathol 130(10) 759–770 https://doi.org/10.1002/cncy.22602 PMID: 35666580
9. Gould AR, Van Arsdall LR, and Hinkle SJ, et al (1983) Tyrosine‐rich crystalloids in adenoid cystic carcinoma: histochemical and ultrastructural observations J Oral Pathol Med 12(6) 478–490 https://doi.org/10.1111/j.1600-0714.1983.tb00360.x
10. Tamgadge S, Tamgadge A, and Satheesan E, et al (2013) Myoepithelial cell – a morphologic diversity – a review Res Rev A J Dent [Internet] 4(1) 5–13 [http://www.stmjournals.com/index.php?journal=RRJoD&page=article&op=view&path%5B%5D=3389%5Cn]
11. Friedmann I, Spilg WG, and Russell TS (1982) Tyrosine crystals in salivary gland tumours J Clin Pathol 35(1) 120 https://doi.org/10.1136/jcp.35.1.120-a PMID: 7061716 PMCID: 497464
12. Valente PT, Hoober JK, and Phillips SJ (1988) Tyrosine-rich crystalloids in pleomorphic adenoma: SEM findings and partial biochemical characterization Ultrastruct Pathol 12(6) 613–620 https://doi.org/10.3109/01913128809056486 PMID: 2853475
13. Martins C, Fonseca I, and Roque L, et al (2005) PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry Mod Pathol 18(8) 1048–1055 https://doi.org/10.1038/modpathol.3800386 PMID: 15920557
14. Napier S (2020) Salivary gland tumours Histopathol Rep Guidel Surg Cancer 185–193 https://doi.org/10.1007/978-3-030-27828-1_16
15. Skalova A, Michal M, and Simpson RHW (2017) Newly described salivary gland tumors Mod Pathol 30(s1) S27–S43 https://doi.org/10.1038/modpathol.2016.167 PMID: 28060365
16. Eveson JW and Cawson RA (1985) Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution J Pathol 146 51–58 https://doi.org/10.1002/path.1711460106 PMID: 4009321
17. Mostand A and Romming C (1973) Crystal structure of L-tyrosine Acta Chem Scand [Internet] 27(2) 401–410 https://doi.org/10.3891/acta.chem.scand.27-0401
18. Mashberg A, Boffetta P, and Winkelman R, et al (1993) Tobacco smoking, alcohol drinking, and cancer of the oral cavity and oropharynx among U.S. veterans Cancer 72(4) 1369–1375 https://doi.org/10.1002/1097-0142(19930815)72:4<1369::AID-CNCR2820720436>3.0.CO;2-L PMID: 8339227
19. Skálová A, Leivo I, and Wolf H, et al (2000) Oncocytic cystadenoma of the parotid gland with tyrosine-rich crystals Pathol Res Pract 196(12) 849–851 https://doi.org/10.1016/S0344-0338(00)80087-4
20. Bellizzi AM and Mills SE (2008) Collagenous crystalloids in myoepithelial carcinoma: report of a case and review of the literature Am J Clin Pathol 130(3) 355–362 https://doi.org/10.1309/J3YVV58V22AKXHWR PMID: 18701407
21. Valko-Rokytovská M, Hubková B, and Birková A, et al (2019) Specific urinary metabolites in malignant melanoma Medicina 55(5) 1–10 https://doi.org/10.3390/medicina55050145
22. Amanda H, Jerad G, and Doug P, et al (2013) Chondroid syringoma (mixed tumor) with tyrosine crystals J Cutan Pathol 40(6) 527 https://doi.org/10.1111/cup.12170
23. Maurice YM, Sheard JD, and Helliwell TR (2009) LETTERS: tyrosine-rich crystalloids in vocal cord mucosa J Clin Pathol 62(1) 95 https://doi.org/10.1136/jcp.2008.061085
24. Lemos LB, Baliga M, and Brister T, et al (1997) Cytomorphology of tyrosine-rich crystalloids in fine needle aspirates of salivary gland adenomas Acta Cytol 41(6) 1709–1713 https://doi.org/10.1159/000333173 PMID: 9390129
25. Carson HJ, Raslan WF, and Castelli MJ, et al (1994) Tyrosine crystals in benign parotid gland cysts: report of two cases diagnosed by fine-needle aspiration biopsy with ultrastructural and histochemical evaluation Am J Clin Pathol 102(5) 699–702 https://doi.org/10.1093/ajcp/102.5.699 PMID: 7942639
26. Reinertsen E, Neill SG, and Nael K, et al (2018) Meningioma with tyrosine-rich crystalloids: a case report and review of the literature Int J Surg Pathol 26(2) 157–160 https://doi.org/10.1177/1066896917727100 PMCID: 5794632
27. Shivashankar VS, Manchiah S, and Govindashetty AM, et al (2019) Tyrosine crystals in nasolabial cyst - a morphologic curiosity diagnosed at cytology Selcuk Tip Derg 3(35) 188–191 https://doi.org/10.30733/std.2019.01309
28. Speight PM and Barrett AW (2020) Salivary gland tumours: diagnostic challenges and an update on the latest WHO classification Diagnostic Histopathol [Internet] 26(4) 147–158 [https://doi.org/10.1016/j.mpdhp.2020.01.001]