Advanced-technique radiation therapy for nasopharyngeal carcinoma in a low resource setting: a review of treatment-related quality of life
Godwin Uwagba1, Adedayo Joseph1,2, Muhammed Habeebu1, Eben Aje1, Aishat Oladipo1,3, Olufunmilayo Fagbemide1, Precious Akowe1,3, Azeezat Ajose1,3, Adebayo Abe1, Samuel Adeneye1,4, Ibrahim Elhamamsi1, Abdallah Kotkat1, Nusirat Adedewe1, Inioluwa Ariyo1, Wonuola Adetugbogbo1 and Francis Durosinmi-Etti1
1NSIA -LUTH Cancer Centre, Lagos University Teaching Hospital, Lagos 100001, Nigeria
2Department of Radiation Biology, Radiotherapy, and Radiodiagnosis, College of Medicine, University of Lagos, Lagos 100001, Nigeria
3Research Department, NSIA-LUTH Cancer Centre, Lagos University Teaching Hospital, Lagos 100001, Nigeria
4College of Medicine, University of Lagos, Lagos 100001, Nigeria
Abstract
Background: Nasopharyngeal carcinoma (NPC) is a rare but significant public health concern, especially in Africa, with a rising global incidence. This study aimed to investigate the pattern of presentation, treatment outcomes and impact on health-related quality of life (HRQOL) of NPC patients at a tertiary institution in Lagos, Nigeria.
Methodology: A retrospective review of all nasopharyngeal cancer patients (n = 125) treated at a tertiary centre in Lagos, Nigeria, from May 2019 to 2022 was done. The European Organisation for Research and Treatment of Cancer (EORTC) H&N 35 questionnaire was used to assess HRQOL at 1-year post treatment and the data were analysed using a statistical package for the social sciences v26.0.
Results: Among 125 patients, the mean age was 46.21 ± 17.82 years with 76% male. Comorbidities were reported in 34 patients (27.2%), smoking history in 18 patients (14.4%) and 50 patients (40%) reported alcohol consumption. Environmental risk factors were identified in six patients (4.8%). The most prevalent histology was squamous cell carcinoma (92.8%), and stage IV was the most common stage (42.4%). Chemoradiation was the primary treatment (63.2%), with intensity-modulated radiotherapy being the most utilised approach (51.2%). Among 125 patients, 51 completed the EORTC questionnaire. Weight loss, sticky saliva, dry mouth, difficulties in swallowing and problems with the sense of taste and smell were the most severe symptoms reported by patients. In the follow-up, 79.2% of patients were reached (50.4% alive, 28.8% deceased). Mortality was significantly associated with age >65 years, weight loss at presentation and consumption of grilled/smoked food.
Conclusion: The study highlights key aspects of NPC in our region including the predominance in males, advanced disease stage at presentation and persistent symptoms post-treatment. Our findings point to the need for targeted initiatives to improve early detection and quality of life for nasopharyngeal patients in the country.
Keywords: nasopharyngeal carcinoma, health-related quality of life, advanced-technique radiation, IMRT, Nigeria
Correspondence to: Adedayo Joseph
Email: djoresearch@gmail.com
Published: 18/09/2024
Received: 26/02/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Nasopharyngeal carcinoma (NPC) has unique characteristics that distinguish it from other epithelial tumours in the head and neck region. According to the International Agency for Research on Cancer (IARC), 133,354 new cases are diagnosed annually, with 72% of cases diagnosed in men [1]. It is a relatively uncommon cancer that accounts for less than 5% of all cancer cases, but with significant morbidity and mortality. In 2020, the IARC recorded eighty thousand nasopharyngeal cancer-related deaths [1]. NPC has a unique geographical distribution, rare in Western countries but endemic in specific regions such as Southeast Asia, North Africa, the Middle East and the Arctic [2–4]. In Africa, it is the 22nd most diagnosed malignancy, with a 5-year prevalence of 22,761 cases across all ages [5]. Historically, Martinson and Aghadiuno [6] documented an increasing incidence of NPC in Nigeria; attributed to a penetrance/pervasiveness of viral infections implicated in its pathogenesis in the region. However, it is difficult to accurately predict the recent national burden because of the predominance of hospital-based studies [7].
The primary cause of NPC is believed to be linked to genetic susceptibility and viral infections with the Epstein-Barr virus (EBV) and, on a much smaller scale, the human papilloma virus [2, 3] EBV which has been linked to NPC has been reported to be an endemic virus in Nigeria [6, 8]. Various environmental and lifestyle factors also contribute to the risk of developing NPC. These include consumption of certain preserved foods, salted fish and foods, alcohol, smoking habits and exposure to certain types of occupational dust and fumes. The interplay between genetic predisposition, viral infection and environmental factors is complex and needs to be fully understood [3, 9]. Frequently, NPC is diagnosed at advanced stages because of its anatomical location and nonspecific early symptoms [10]. In particular, patients in Sub-Saharan Africa present with advanced-stage disease as a result of a combination of non-specific symptoms and other healthcare infrastructural and workforce deficiencies [11–14]. Stage at presentation is a crucial determinant for treatment-related quality of life specifically during radiotherapy and, ultimately, survival outcomes [15, 16].
Radiotherapy is the primary treatment modality for NPC, with intensity-modulated radiotherapy (IMRT) being the preferred technique based on data showing that it reduces toxicity and improves target coverage by ensuring precise targeting while minimizing doses to surrounding normal tissue [17, 18]. Chemotherapy, as a neo-adjuvant, concurrent or adjuvant treatment, has been shown to improve regional control and overall survival outcomes in patients compared to the historical use of chemotherapy only in late-stage and metastatic diseases [19–21].
Nasopharyngeal cancer substantially impacts patients’ health-related quality of life (HRQOL) due to its distinctive anatomical location, resulting in symptoms and treatment-related side effects [22]. The location of the nasopharynx adjacent to essential structures in the head and neck region (brain, ears, salivary glands and optic nerve) puts these structures at risk of disease extension/infiltration and normal tissue damage from inclusion in the radiotherapy field. Long-term adverse effects such as hearing loss, speech defects, difficulty swallowing, xerostomia (dry mouth) and cognitive changes, which may persist and affect day-to-day life are common with NPC treatment [23]. Literature shows that treatment-related adverse effects such as dysphagia, dry mouth and mouth-opening difficulties profoundly impact the quality of life negatively [24, 25]. In recent times, advanced treatment techniques and the possibility of re-planning during treatment based on repeated imaging are beneficial to patients’ overall quality of life and survival [25, 26]. Understanding this impact is crucial for healthcare professionals to provide comprehensive care and support to patients affected by the disease. This study aimed to investigate the treatment outcomes and impact on HRQOL, among patients with NPC at a tertiary institution in Lagos, Nigeria.
Methods
Study design and patient selection
A retrospective analysis was conducted on 125 adult patients with histologically diagnosed NPC at the NSIA-LUTH Cancer Center, Lagos University Teaching Hospital (LUTH), Nigeria, over 3 years starting from May 2019 to 2022. Data on sociodemographic characteristics, clinical history, treatment modalities and radiotherapy dose details, HRQOL documented at 1-year post treatment follow up visit were obtained from clinical case files and patients’ electronic medical records. Verbal informed consent was obtained from participants before follow-up. Ethical consideration was obtained from the LUTH Health Research and Ethics Committee. Patient information was anonymized and assigned unique identifiers prior to statistical analysis.
Treatment
According to the cancer staging manual by the American Joint Committee on Cancer, eighth edition, disease staging was categorised by location and Tumour, Node, and Metastasis (TNM) criteria to stages I–IVA and IVB. The treatment protocol at our institution, following current guidelines, involves conformal radiotherapy (IMRT or volumetric modulated arc therapy (VMAT)) for all localised and locally advanced NPC, with chemotherapy (neo-adjuvant, concurrent and adjuvant) administered as indicated per National Comprehensive Cancer Network guidelines. Patients with disseminated metastatic disease receive systemic treatment only or best supportive care based on performance status.
Quality of life instrument
The European Organisation for Research and Treatment of Cancer (EORTC) H&N 35 questionnaire was used to assess these patients’ HRQOL [27]. This is a validated tool used to measure the impact of head and neck cancers on patients’ quality of life from their perspective. The QLQ-H&N35 comprises seven multi-item scales that assess pain, swallowing, taste and smell senses, speech difficulties, social eating, social contact and physical appearance. Furthermore, eleven single items survey problems with teeth, mouth opening, dry mouth, sticky saliva, coughing, feeling ill, use of pain medications, nutritional supplements or a nasogastric tube and weight changes (gain or loss) [27]. In this study, QLQ-H&N35 was administered to patients by an interviewer within a 2-week duration while assessing survival outcomes.
Follow-up
After the completion of treatment, patients were followed up with a routine clinical consultation, physical examination and imaging of the head and neck (nasopharynx) and other body parts as indicated. Our centre routinely collects data during patient visits at different time points for patients with head and neck cancers, including NPC to guide treatment. For this study, we report on the HRQOL data collected at the 1-year post treatment date for our nasopharyngeal cancer patients. This data were gathered either in-person during the patient’s scheduled follow-up visit or via telephone within 2 weeks of the 1-year follow-up date by trained centre staff, ensuring all patients were assessed within a consistent timeframe. Each patient’s 1-year follow-up point was personalised based on the date of their completion of radiation therapy. Thus, the 1-year assessments occurred on different dates specific to each patient’s treatment timeline. Verbal consents were obtained before each consultation. Loss to follow-up was determined as any patient who missed three consecutive follow-up appointments and was unreachable via other provided forms of communication, in which case survival status was determined by records from the last clinic visit.
Statistical analysis
THE EORTC H&N35 scoring manual was used to calculate the score to range from 0 to 100 [27]. Data were analysed using a statistical package for the social sciences 26.0 statistics software. Data were presented in frequencies, percentages, tables and means with standard deviations. Mann–Whitney U and Kruskal–Wallis tests were used to examine associations between different quantitative variables and the significance level was set at p < 0.05.
Results
One hundred and twenty-five patients, with a mean age of 46.21 ± 17.82 years (range, 9–97) were treated for NPC in the study institution within the 3-year duration. The predominant gender was male (76%). Patient characteristics are summarized in Table 1.
Prevalent comorbidities were reported in 34 patients (27.2%). Of these, 26 patients had hypertension alone, 7 had co-existing hypertension and type 2 diabetes mellitus and 1 had type 2 diabetes mellitus. Smoking history was present in 18 patients (14.4%) and 50 patients (40%) reported alcohol consumption while 14.4% reported both alcohol consumption and smoking. Environmental risk factors were identified in six patients (4.8%). The most prevalent histology was squamous cell carcinoma (92.8%) and stage IV was the most common stage (Table 2). Overall, following institutional treatment protocols, 15 patients (12%) had a prior surgical intervention, 79 patients (63.2%) were treated with chemoradiation and 29 patients (23.2%) had radiotherapy alone,
with IMRT being the most utilised approach (51.2%) and VMAT was used for 41.6%. A total of 15 patients (12.0%) in this study population have had surgery. Of these, 14 (11.2%) had chemoradiation in addition to the surgical procedure, while only 1 (0.8%) had chemotherapy together with surgery.
Table 1. Sociodemographic and patient characteristics.
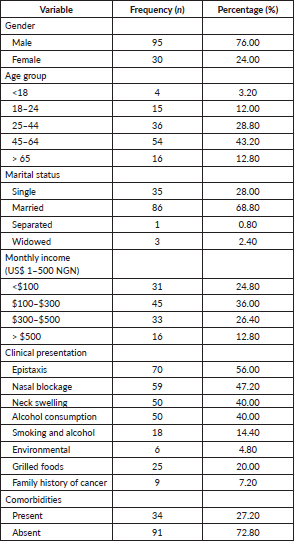
Table 2. Tumour and treatment characteristics.
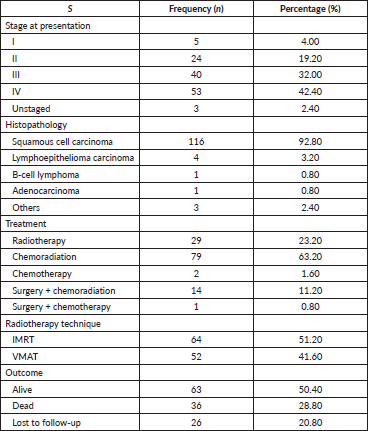
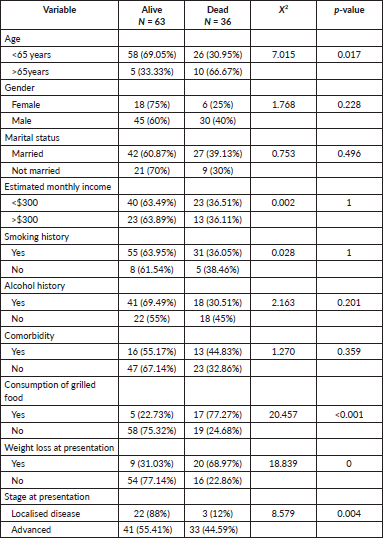
Among the 125 patients, 51 completed the EORTC questionnaire (Table 3). In the H&N-35 module, weight loss, sticky saliva, dry mouth, difficulties in swallowing and problems with the sense of taste and smell were the most severe symptoms reported by patients. In the follow-up, 99 patients were reached (63 alive, 36 deceased). Mortality at 1 year was significantly associated with age >65 years, weight loss at presentation and dietary consumption of grilled/smoked food (Table 4).
Table 3. HRQOL scores (QLQ-H&N-35).
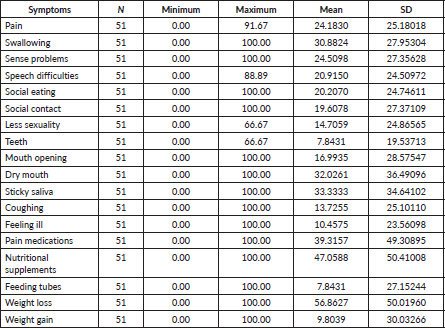
Table 4. Factors associated with poor outcomes among NPC patients (n = 99).
Discussion
This article describes the clinicopathological characteristics, treatment outcomes and quality of life at 1-year post treatment of patients with nasopharyngeal cancer in Lagos, Nigeria. The male-to-female ratio in this study was 3.2:1. This correlates with several studies from all over the world, including African countries, which show that NPC predominantly affects the male gender [15, 22, 28–30]. Most patients (43.2%) in this study were 45–64 years old. Age group distribution of NPC typically varies between endemic and non-endemic regions. In a tertiary hospital in Indonesia, most patients diagnosed were within the 41–50 age group [30]. Also, in the Asian high-risk population, the incidence of NPC peaks between ages 45–59 years. Meanwhile, it is mainly found among the young adult population in non-endemic regions [3]. The clinical presentation of NPC largely depends on the extent of the disease spread anatomically. Thus, early stage diseases usually present with non-specific symptoms leading to missed and delayed diagnoses [2, 4, 31]. All patients in this study had initially presented at other centres. Thus, at a presentation at our institution, they had typical symptoms of advanced disease spread, such as epistaxis, nasal blockage, neck swelling (nodal involvement), hearing impairment (cranial nerve deficit), recurrent upper respiratory tract infection (URTI), headache, weight loss, dysphagia, visual impairment, hoarseness and mouth swelling, with epistaxis having the highest frequency (56%). This corresponds with other Nigerian studies which showed similar clinical presentations at tertiary institutions [28, 29].
Tobacco smoking, alcohol consumption and consumption of grilled foods were the common potential risk factors identified among this study population, with environmental exposure to toxic chemicals being the least (4.8%). We identified exposure to potential environmental toxins in six patients; some of the toxins include construction materials, metal and wood dust, motor fuels, acetone, bromophol fumes and paint products. In this study, a vast majority of the patients presented with advanced disease stages, that is, stages III (32%) and IV (42.4%), respectively, while the most common histology finding was squamous cell carcinoma (92.8%). The late stage at diagnosis is a relatively common feature of NPC in both endemic and non-endemic regions because of its non-specific presentation in the early stages [2, 4]. Similar to the findings in this study, Cengiz et al [23] also observed that 75% of cases in his study population presented with an advanced stage. Also, 54% of the patients in a single institution study in Indonesia presented with stage IV disease [30]. With these similar figures from different parts of the world, the survival outcome rates would be expected to follow the same trend, but that is not the case. The difference in healthcare infrastructures and the availability of quality cancer care have been postulated to be the drivers for higher mortality rates in low and middle-income countries.
According to the World Health Organisation, NPC is classified into three histological subtypes: Keratinizing squamous cell carcinoma, non-keratinizing differentiated and undifferentiated NPC [32]. From the histological classification of patients in this study, 36.0% had been diagnosed with undifferentiated NPC. This finding, along with similar observations of predominantly NPC cases of the non-keratinizing histology type in other centres across Nigeria (Ibadan, Ilorin and Kaduna), further proves that an increase in NPC burden can be linked to EBV infections [28, 29]. All 15 patients who underwent surgery had previous interventions outside our centre, which primarily offers chemotherapy and radiotherapy services. Specifically, three patients had elective tracheostomies, seven patients had endoscopic sinus surgery aimed at tumour excision, and one patient had undergone nasal polypectomies before the presentation. Additionally, four patients had a history of various other surgeries, including herniorrhaphy orchidectomy and neck surgery.
Radiotherapy treatment of NPC requires a wide treatment field that may affect several vital structures such as the parotid gland, muscles of mastication, temporomandibular joint and other neural structures [23, 33]. Advancements in cancer treatment modalities have improved survival outcomes for NPC patients. However, the increase in long-term survival has led to a higher burden of long-term toxicities that impact patients’ quality of life. In our study, the findings from the EORTC QLQ-H&N35 questionnaire showed that weight loss, sticky saliva, dry mouth, difficulties in swallowing and problems with the sense of taste and smell were the most severe symptoms reported by patients [25]. These symptoms are primarily related to xerostomia, a condition frequently cited as one of the most prevalent complications following radiotherapy for head and neck cancers. Our results align closely with those reported by Fang et al [25], who also identified dry mouth and sticky saliva as predominant symptoms in their cohort. Similarly, Huang et al [33], in a longitudinal study, observed that symptoms related to swallowing, taste, mouth opening, dry mouth and sticky saliva remained significantly worse at 1-year post-treatment in patients with nasopharyngeal cancer. Our findings suggest that the impacts of NPC and its treatment on quality of life are profound and persist long after the initial treatment period has ended. Our study shows a need to integrate supportive care and symptom management for NPC patients during and after treatment. Additionally, there should be an emphasis on nutritional support and counseling, as weight loss and swallowing difficulties can lead to significant nutritional deficiencies, further impacting the patient’s recovery and quality of life.
79.2% (99) of patients had follow-up calls and 20.8% (26) were lost to follow-up. Of the 99 patients who were reached, 50.4% were alive and the remaining 28.8% were reported dead. Mortality was significantly associated with age >65, weight loss at presentation and dietary consumption of grilled/smoked food. Our limitations include a primary focus on the quality of life assessments at the 1-year post-treatment mark, with only a preliminary overview of overall survival rates provided. Detailed analyses of disease control and progression-free survival were not included in this study. The focus of our next research will be to extend our follow-up with these patients, aiming to report on these critical outcomes up to 5 years post-treatment. This expanded study will provide a comprehensive view of long-term survival and disease control, which will be invaluable for understanding outcomes for NPC in low-resource settings. Additionally, while our current study did not investigate the impact of local habits or genetic factors, these represent significant variables that could influence outcomes. Future studies should explore how local habits and genetic predispositions may uniquely affect our patient population.
Conclusion
Despite advancements in radiotherapy that have improved survival rates, NPC continues to present significant challenges due to late-stage diagnosis and the resultant severe long-term toxicities that adversely affect quality of life. Our findings reveal that symptoms such as weight loss, sticky saliva, dry mouth and swallowing difficulties are prevalent and persist long after treatment and this shows the need for integrated supportive care and comprehensive symptom management for these patients during and after treatment. Long-term follow-up important to document the effects of treatment on quality of life and survival and allow clinicians to identify interventions that mitigate long-term toxicities and improve overall patient quality of life and survival outcomes.
List of abbreviations
EBV, Epstein-Barr virus; EORTC, European Organization for Research and Treatment of Cancer; H&N, Head and neck; HRQOL, Health-related quality of life; IMRT, Intensity-modulated radiation therapy; NPC, Nasopharyngeal carcinoma; NSIA-LUTH, Nigerian Sovereign Investment Authority-Lagos University Teaching Hospital; URTI, Upper respiratory tract infection; VMAT, Volumetric modulated arc therapy.
Acknowledgments
The authors would like to acknowledge the management and staff of the NSIA-LUTH Cancer Center for providing the facilities and resources necessary for this research. The authors would also like to acknowledge all study participants from the Centre.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript; and no other relationships or activities that could appear to have influenced the submitted work.
Funding
This study was funded internally by the NSIA-LUTH Cancer Center, Lagos, Nigeria.
Author contributions
All authors contributed to the conception and design of the study.
Godwin Uwagba, Adedayo Joseph, Olufumilayo Fagbemide and Azeezat Ajose contributed to the data analysis of the work.
All authors contributed to the drafting and revision of the manuscript.
All authors approved the final version for publication.
All authors agree to be accountable for all aspects of the work.
Adedayo Joseph is the corresponding author and provided overall leadership for all aspects of the study.
Data sharing statement
Supporting information is available from the corresponding author on request.
References
1. International Agency for Research on Cancer (n.d.) Estimated Number of New Cases in 2020, World, Both Sexes, All Ages (Excl. NMSC) Cancer Today http://gco.iarc.fr/today/home Data accessed: 05/07/23
2. Chua MLK, Wee JTS, and Hui EP, et al (2016) Nasopharyngeal carcinoma Lancet 387 1012–1024 https://doi.org/10.1016/S0140-6736(15)00055-0
3. Chang ET, Ye W, and Zeng Y-X, et al (2021) The evolving epidemiology of nasopharyngeal carcinoma Cancer Epidemiol Biomarkers Prev 30 1035–1047 https://doi.org/10.1158/1055-9965.EPI-20-1702 PMID: 33849968
4. Chen Y-P, Chan ATC, and Le Q-T, et al (2019) Nasopharyngeal carcinoma Lancet 394 64–80 https://doi.org/10.1016/S0140-6736(19)30956-0 PMID: 31178151
5. Cancer (IARC) (n.d.) TIA for R on. Global Cancer Observatory https://gco.iarc.fr/ Data accessed: 14/11/22
6. Martinson FD and Aghadiuno PU (1984) Nasopharyngeal cancer in Nigeria IARC Sci Publ 63 501–511
7. da Lilly-Tariah OB, Somefun AO, and Adeyemo WL (2009) Current evidence on the burden of head and neck cancers in Nigeria Head Neck Oncol 1 14 https://doi.org/10.1186/1758-3284-1-14 PMID: 19476614 PMCID: 2694192
8. Diakite M, Shaw-Saliba K, and Lau C-Y (2023) Malignancy and viral infections in Sub-Saharan Africa: a review Front Virol Lausanne Switz 3 1103737 https://doi.org/10.3389/fviro.2023.1103737
9. Song Y, Cheng W, and Li H, et al (2022) The global, regional, national burden of nasopharyngeal cancer and its attributable risk factors (1990–2019) and predictions to 2035 Cancer Med 11 4310–4320 https://doi.org/10.1002/cam4.4783 PMID: 35475595 PMCID: 9678109
10. Howlett J, Hamilton S, and Ye A, et al (2021) Treatment and outcomes of nasopharyngeal carcinoma in a unique non-endemic population Oral Oncol 114 105182 https://doi.org/10.1016/j.oraloncology.2021.105182 PMID: 33503570
11. Garandawa HI, Ahmad BM, and Nggada HA (2009) Nasopharyngeal cancer in North--Eastern Nigeria: clinical trends Niger J Clin Pract 12 379–382
12. Iseh K, Abdullahi A, and Malami S (2009) Clinical and histological characteristics of nasopharyngeal cancer in Sokoto, North Western, Nigeria West Afr J Med 28 151–155 https://doi.org/10.4314/wajm.v28i3.48438
13. Yates SM, Yawale I, and Aliyu AS, et al (2018) Nasopharyngeal carcinoma at the Ahmadu Bello University Teaching Hospital Zaria: a 22-year histopathological review (1992–2013) Arch Med Surg 3(1) 24–29 https://doi.org/10.4103/archms.archms_10_18
14. Ngwa W, Addai BW, and Adewole I, et al (2022) Cancer in sub-Saharan Africa: a lancet oncology commission Lancet Oncol 23 e251–e312 https://doi.org/10.1016/S1470-2045(21)00720-8 PMID: 35550267 PMCID: 9393090
15. Fang F-M, Chien C-Y, and Tsai W-L, et al (2008) Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy—a longitudinal study Int J Radiat Oncol 72 356–364 https://doi.org/10.1016/j.ijrobp.2007.12.054
16. Liao K-C, Chuang H-C, and Chien C-Y, et al (2021) Quality of life as a mediator between cancer stage and long-term mortality in nasopharyngeal cancer patients treated with intensity-modulated radiotherapy Cancers 13 5063 https://doi.org/10.3390/cancers13205063 PMID: 34680211 PMCID: 8533735
17. Wong KCW, Hui EP, and Lo K-W, et al (2021) Nasopharyngeal carcinoma: an evolving paradigm Nat Rev Clin Oncol 18 679–695 https://doi.org/10.1038/s41571-021-00524-x PMID: 34194007
18. Tuan JKL, Ha TC, and Ong WS, et al (2012) Late toxicities after conventional radiation therapy alone for nasopharyngeal carcinoma Radiother Oncol 104 305–311 https://doi.org/10.1016/j.radonc.2011.12.028 PMID: 22280806
19. Lee AWM, Tung SY, and Chua DTT, et al (2010) Randomized trial of radiotherapy plus concurrent–adjuvant chemotherapy vs. radiotherapy alone for regionally advanced nasopharyngeal carcinoma JNCI J Natl Cancer Inst 102 1188–1198 https://doi.org/10.1093/jnci/djq258
20. Chen Y, Sun Y, and Liang S-B, et al (2013) Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China Cancer 119 2230–2238 https://doi.org/10.1002/cncr.28049 PMID: 23576020
21. Gamal AH (2021) Epidemiology and outcomes of nasopharyngeal cancer Pharynx Diagnosis and Treatment, BoD—Books on Demand pp 43–51
22. Alsafadi N, Alqarni M, and Attar M, et al (2020) Nasopharyngeal cancer: prevalence, outcome, and impact on health-related quality of life at Princess Norah Oncology Center, Jeddah, Saudi Arabia Cureus 12 e8199 https://doi.org/10.7759/cureus.8199 PMID: 32572356 PMCID: 7302723
23. Cengiz M, Özyar E, and Esassolak M, et al (2005) Assessment of quality of life of nasopharyngeal carcinoma patients with EORTC QLQ-C30 and H&N-35 modules Int J Radiat Oncol 63 1347–1353. https://doi.org/10.1016/j.ijrobp.2005.05.057
24. Lovell SJ, Wong H-B, and Loh K-S, et al (2005) Impact of dysphagia on quality-of-life in nasopharyngeal carcinoma Head Neck 27 864–872 https://doi.org/10.1002/hed.20250 PMID: 16114007
25. Fang F-M, Tsai W-L, and Lee T-F, et al (2010) Multivariate analysis of quality of life outcome for nasopharyngeal carcinoma patients after treatment Radiother Oncol 97 263–269 https://doi.org/10.1016/j.radonc.2010.05.022 PMID: 20817290
26. Yang H, Hu W, and Wang W, et al (2013) Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma Int J Radiat Oncol 85 e47–e54 https://doi.org/10.1016/j.ijrobp.2012.09.033
27. EORTC (n.d.) Head and Neck Cancer (update of QLQ-H&N35)—EORTC—Quality of Life: EORTC—Quality of Life https://qol.eortc.org/questionnaire/qlq-hn43/ Date accessed: 09/07/23
28. Alabi BS, Badmos KB, and Afolabi OA, et al (2010) Clinico-pathological pattern of nasopharyngeal carcinoma in Ilorin, Nigeria Niger J Clin Pract 13 445–8
29. Ogun GO, Olusanya AA, and Akinmoladun VI, et al (2020) Nasopharyngeal carcinoma in Ibadan, Nigeria: a clinicopathologic study Pan Afr Med J 36 82 https://doi.org/10.11604/pamj.2020.36.82.19657 PMID: 32774641 PMCID: 7392876
30. Handayani R, Afriani Dewi Y, and Madani DZ (2020) Prevalence of nasopharyngeal carcinoma patients in departement of orl-hns hasan sadikin general hospital 2010–2017 Int J Nasopharyngeal Carcinoma IJNPC 2 01–03 https://doi.org/10.32734/ijnpc.v2i01.3191
31. Wang KH, Austin SA, and Chen SH, et al (2017) Nasopharyngeal carcinoma diagnostic challenge in a nonendemic setting: our experience with 101 patients Perm J 21 16–180 https://doi.org/10.7812/TPP/16-180 PMID: 28609261 PMCID: 5469434
32. Stelow EB and Wenig BM (2017) Update from the 4th edition of the world health organization classification of head and neck tumours: nasopharynx Head Neck Pathol 11 16–22 https://doi.org/10.1007/s12105-017-0787-0 PMID: 28247232 PMCID: 5340728
33. Huang T-L, Tsai M-H, and Chuang H-C, et al (2020) Quality of life and survival outcome for patients with nasopharyngeal carcinoma treated by volumetric-modulated arc therapy versus intensity-modulated radiotherapy Radiat Oncol Lond Engl 15 84 https://doi.org/10.1186/s13014-020-01532-4





