Prevalence and treatment of human epidermal growth factor receptor 2-altered non-small cell lung cancer: a retrospective analysis and systematic literature review
Ning Yi Yap1,2, Komathi Perumal1,3 and Pathmanathan Rajadurai1,2,4
1Laboratory, Subang Jaya Medical Centre, Subang Jaya 47500, Selangor, Malaysia
2Jeffrey Cheah School of Medicine & Health Sciences, Monash University, Bandar Sunway, Petaling Jaya 47500, Selangor, Malaysia
3ePink Health Sdn. Bhd., Shah Alam 40150, Selangor, Malaysia
4Faculty of Medicine, Universiti Malaya, Kuala Lumpur 50603, Malaysia
Abstract
Human epidermal growth factor receptor 2 (HER2) is known for its oncogenic activities in diverse cancers, including non-small cell lung cancer (NSCLC). However, the prevalence of HER2 alterations in Malaysian NSCLC patients remains unreported. This study examined the prevalence and characteristics of HER2 mutations and amplification in a Malaysian cohort. Additionally, a systematic review was conducted to evaluate the global prevalence of HER2 alterations in NSCLC, as well as the efficacy of HER2-targeted therapies observed in clinical trials. NSCLC tumour samples received from October 2019 to December 2022 for next-generation sequencing diagnostics were included in the retrospective analysis. In this patient cohort, HER2 alteration was present in 5.8% of patients; 3.9% had HER2 mutations, 1.5% had HER2 amplifications and 0.4% were both HER2-mutated and amplified. HER2 exon 20 insertions were the most common HER2 variants, detected in 47/59 (79.7%) of HER2-mutated patients. Among cases with HER2 exon 20 insertions, the Y772_A775dup variant was found in 34 patient samples. HER2-mutated patients were significantly younger than non-HER2-mutants (61 versus 64 years old; p = 0.046) and were inclined to be female and never-smokers, albeit not statistically significant. Patients with HER2 amplification were more likely to have progressed post-tyrosine kinase inhibitor therapy (p = 0.015). The systematic review highlighted a global variation in the prevalence of HER2 alterations in NSCLC, ranging from 0.3% to 9.1% for mutations and 0.2% to 19% for amplification. Finally, phase II clinical trials involving HER2-altered NSCLC patients demonstrated promising treatment outcomes with trastuzumab deruxtecan, trastuzumab emtansine, pyrotinib, pyrotinib + apatinib and trastuzumab + pertuzumab + docetaxel. In conclusion, the prevalence of HER2 alteration among Malaysian NSCLC patients falls within the global range. A systematic review of clinical trials revealed promising treatment outcomes and Malaysian NSCLC patients with HER2 alterations are anticipated to similarly benefit from HER2-targeted therapies.
Keywords: HER2, Malaysia, next-generation sequencing, non-small cell lung cancer, Southeast Asia
Correspondence to: Pathmanathan Rajadurai
Email: drpathma@gmail.com
Published: 01/08/2024
Received: 28/05/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Human epidermal growth factor receptor 2 (HER2), also known as ErbB2, is a transmembrane glycoprotein receptor exhibiting intracellular tyrosine kinase activity [1]. It consists of extracellular, transmembrane and intracellular domains, and plays important roles in various cellular functions including adhesion, differentiation, growth, apoptosis and migration [2, 3]. HER2 has garnered considerable attention due to its role in tumorigenesis and potential as a therapeutic target [3, 4]. Its aberrations have been implicated in the development and progression of various cancers, including non-small cell lung cancer (NSCLC) [3].
Three types of HER2 oncogene activating mechanisms have been described in cancers, and they include gene mutation, gene amplification and protein overexpression [3]. These mechanisms of HER2 activation have significant implications on treatment strategies, and prognostic outcomes which may differ according to the cancer type. HER2 amplification and overexpression are well-established predictive markers for response to HER2-targeted monoclonal antibodies such as trastuzumab, in patients with breast and gastric cancers [2, 5]. However, HER2 protein overexpression has not demonstrated reliability in identifying NSCLC patients who may benefit from HER2-targeted therapies [6, 7]. In contrast, HER2 mutations have shown greater promise in selecting NSCLC patients who are likely to respond to HER2-targeted therapies [8].
Currently, standard chemotherapy or immunotherapy is administered to patients with HER2-mutant NSCLC, but their effectiveness as second- or later-line treatment is limited [9]. Nonselective tyrosine kinase inhibitors (TKIs) have shown limited benefit in NSCLC patients with HER2 mutation, with objective response rates (ORRs) ranging from 0% to 19% [10]. Trastuzumab-based chemotherapy was not found to be superior to chemotherapy alone whereas selective HER2 TKIs (e.g., poziotinib and pyrotinib) showed better activity in pre-treated NSCLC patients with HER2 mutation [10]. More favourable data were reported in phase II studies evaluating antibody-drug conjugates (ADC) ado-trastuzumab emtansine and trastuzumab deruxtecan in HER2-mutated NSCLC patients [11, 12]. These agents bring hope to the management of HER2-altered NSCLC.
Malaysia is a multi-ethnic Southeast Asian country, comprising an ethnic Malay majority, as well as significant Chinese, Indian and indigenous populations. Lung cancer survival is the worst among all cancers in Malaysia; the overall 5-year relative survival for lung, trachea and bronchus cancer among Malaysian patients was 11.0%, with a median survival time of 6.8 months [13]. The prevalence of HER2 mutation and amplification among NSCLC patients is not well-reported in the Southeast Asia region, and has not been reported in Malaysia. Therefore, we have performed a retrospective study to elucidate the prevalence of HER2 alterations and the characteristics of NSCLC patients with these alterations, based on diagnostic next-generation sequencing (NGS) performed at a tertiary private referral medical center in Malaysia. Additionally, a systematic literature review was conducted to offer a comprehensive overview of the existing evidence concerning the prevalence of HER2 mutation/amplification in NSCLC, as well as the efficacy of HER2-targeted therapies observed in prospective clinical trials involving NSCLC patients with HER2 alterations.
Methods
Determining prevalence of HER2 mutations and amplification among NSCLC patients in Malaysia.
Patient samples
Tumour samples from lung cancer patients from several medical centers in Malaysia were collected and sent to the Subang Jaya Medical Centre (SJMC) laboratory for NGS. We analyzed the NGS results of consecutive samples received from October 2019 to December 2022, to determine the prevalence of HER2 mutations and amplification. Ethical committee approval for the analysis of the retrospective NGS data was granted by the SJMC ethics committee (Ref: 201907.3 and Ref: 202109.3).
NGS testing
DNA from samples received from October 2019 to 2020 was sequenced using the Ion AmpliSeq Colon and Lung Cancer Research Panel v2 (Thermo Fisher Scientific, Waltham, MA, USA), while ribonucleic acid (RNA) was sequenced using the Ion AmpliSeq RNA Fusion Lung Cancer Research Panel or the Oncomine Focus Assay (Thermo Fisher Scientific, Waltham, MA, USA). DNA and RNA from samples received from November 2020 to December 2022 were sequenced using the Oncomine Precision Assay GX (Thermo Fisher Scientific, Waltham, MA, USA). The NGS testing process followed the method previously described by Rajadurai et al [14]. HER2 mutations and amplification were detected using the targeted NGS panels.
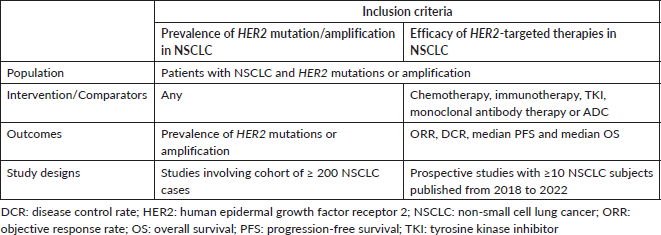
Table 1. Criteria for considering studies for systematic review, based on the population, intervention, comparator and PICOS structure.
Statistical analysis and data visualization
Statistical analysis was performed using the IBM SPSS Statistics Version 22.0 (IBM Corporation, Armonk, NY, USA). Patients’ demographic and clinical characteristics were evaluated using Pearson’s chi-square test or Fisher’s test for categorical variables, while the Mann-Whitney test was employed for comparing the patients’ age. The HER2 mutation lollipop diagram was generated using the MutationMapper visualization tools available at the cBioPortal site (https://www.cbioportal.org/) [15].
Systematic review of HER2 mutations and amplification in NSCLC
The systematic literature review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [16].
Search strategy
A systematic literature review was conducted to obtain (a) the prevalence of HER2 mutation/amplification in NSCLC, and (b) the efficacy of HER2-targeted therapies in NSCLC patients with HER2 alterations. A comprehensive database search was performed in PubMed and Web of Science to identify relevant studies published up to 31 December 2022. The Medical Subject Headings and text word search terms used were (‘HER2’ or ‘HER-2’ or ‘ERBB2’ or ‘ERBB-2’) AND (‘lung cancer’ or ‘NSCLC’). The studies were screened by two reviewers (NYY and KP) based on the article titles, abstracts and contents. The general inclusion criteria used to evaluate records include articles or abstracts published in the English language, and studies in which NSCLC patients were included. Studies involving only in vitro or in vivo samples, and review-type articles were excluded. The article selection criteria based on the populations, interventions and comparators, outcomes and study design (PICOS) are shown in Table 1.
Specific inclusion and exclusion criteria were also defined according to the two subtopics of the systematic literature review, as follows: (a) For systematic review on the prevalence of HER2 mutations and amplification, publications reporting the prevalence of HER2 mutations and amplification from a cohort of ≥ 200 NSCLC cases were included. Analyses involving only epidermal growth factor receptor (EGFR), Kirsten rat sarcoma 2 viral oncogene homologue (KRAS), anaplastic lymphoma kinase (ALK) or ROS proto-oncogene 1 (ROS1) wild-type cases, analyses using liquid biopsy samples only and reports of HER2 protein overexpression were excluded. (b) For a systematic review of clinical trials evaluating the efficacy of treatment in NSCLC patients with HER2 mutations or amplification, prospective studies with ≥10 NSCLC subjects that were published from 2018 to 2022 were included. Retrospective studies and case series were excluded.
Data extraction
Data were extracted from articles that met the defined inclusion and exclusion criteria by one independent reviewer, and verified by a second reviewer. Data extracted for two subtopics of the systematic literature review were as follows: (a) For systematic review on the prevalence of HER2 mutations or amplification, data extracted were on the prevalence of HER2 mutations and amplification. (b) For a systematic review on prospective clinical trials evaluating the efficacy of treatment in NSCLC patients with HER2 mutations or amplification, data extracted were the ORR, disease control rate (DCR), median progression-free survival (PFS) and median overall survival (OS).
Results
Prevalence of HER2 mutations and amplification in Malaysian NSCLC patients
Patient cohort
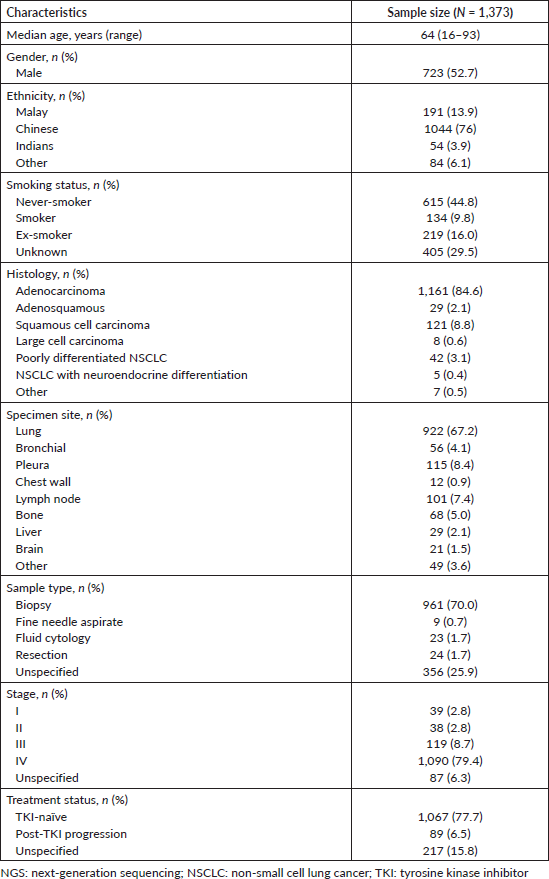
The demographic features of 1,373 NSCLC patients whose tumour samples were analyzed at the SJMC laboratory are shown in Table 2. Approximately half of the patients were male (52.7%), and the median patient age was 64 years old (range 16–93 years old). Most of the patients were of Chinese descent (76%), and nearly half of the patients were never smokers (44.8%). Most tumours (84.6%) were adenocarcinomas, and 88.1% of patients had advanced stage NSCLC (stage III or IV disease). Most patients (77.7%) were TKI-naïve.
Table 2. Clinical characteristics of Malaysian NSCLC patients whose tumour samples were analysed using NGS from October 2019 to December 2022.
NGS and HER2 profile of NSCLC patient cohort
Among the NSCLC specimens analysed at our centre, nearly half (n = 627, 45.7%) showed EGFR alteration, followed by KRAS alteration (174, 12.7%) and ALK alteration (85, 6.2%). HER2 alteration was present in 79 patients (5.8%); 54 (3.9%) of these were HER2 mutations only, 20 (1.5%) were HER2 amplification only and 5 (0.4%) were both HER2-mutated and amplified (Figure 1a). The HER2 mutation variants reported in the NSCLC patients are shown in Figure 1b. HER2 exon 20 insertions, found in the TKI domain, were the most common HER2 variants among these NSCLC patients, with 47 out of 59 (79.7%) patients having this form of HER2 alteration. The HER2 exon 20 insertion Y772_A775dup was the most frequent HER2 variant, found in 34 patient samples. Other HER2 mutations are found in the extracellular ligand binding domain (S310S/Y, 7 patients) and transmembrane domain (V659E, 2 patients).
HER2 exon 20 insertions were mutually exclusive with ALK, BRAF, EGFR, RET, ROS1 and MET genetic alterations. Two patients with HER2 Y772_A775dup harboured KRAS alterations, one with KRAS amplification and the other KRAS K117N. EGFR sensitising mutations were detected in four patients with HER2 S310S/Y variants; of these, two patients were post-TKI progression cases. In addition, 11 patients with HER2 amplification had EGFR sensitising mutation; of these, 5 patients were post-TKI progression. TP53 mutations were the most common co-mutations seen with HER2 mutation (14 patients) and HER2 amplification (9 patients).
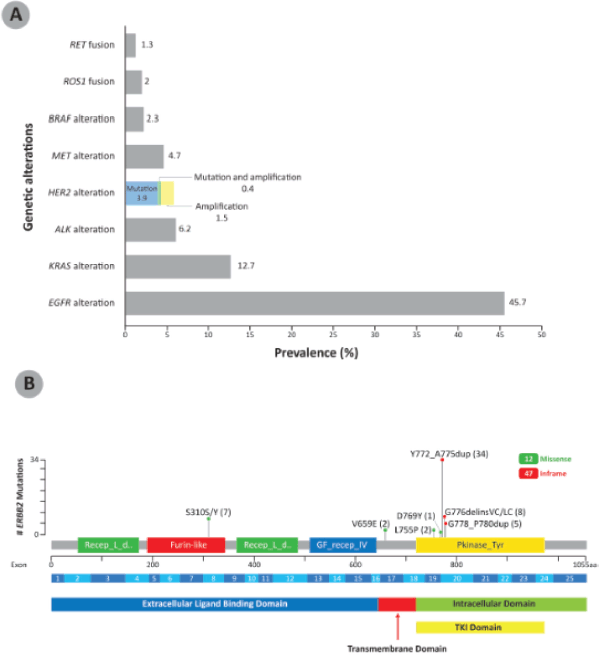
Figure 1. (a): Prevalence of genetic alterations in a cohort of NSCLC patients in Malaysia (N = 1,373). The breakdown of prevalence of HER2 mutation, amplification, as well as mutation and amplification are shown in color. (b): HER2 mutation variants reported in NSCLC patients in Malaysia (n = 59). The number of patients showing the specific mutations are indicated in brackets. HER2 exon 20 insertions were the most common HER2 variant in the NSCLC patients. BRAF: B-Raf proto-oncogene; MET: mesenchymal-epithelial transition; RET: RET proto-oncogene.
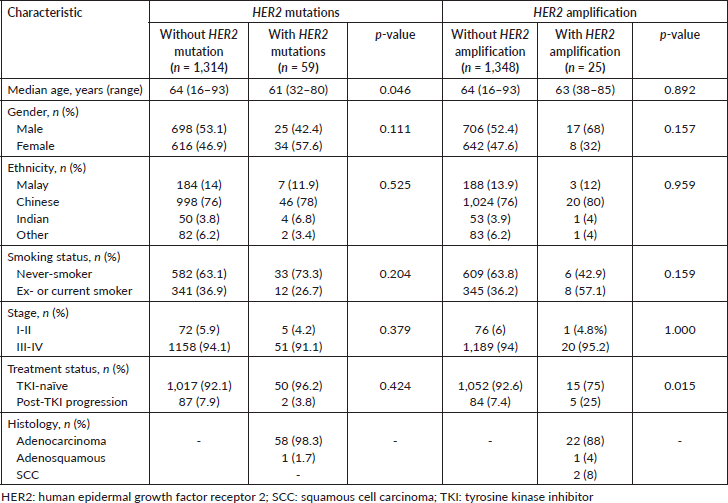
Characteristics of patients with HER2 mutations and amplification
Patients with HER2 mutations were significantly younger than non-HER2-mutants (median age 61 versus 64 years old; p = 0.046), and were inclined to be female and never-smokers (not statistically significant; p = 0.111 and 0.204, respectively) (Table 3). On the other hand, patients with HER2 amplification were inclined to be male, and ex- or current smokers (not statistically significant; p = 0.157 and p = 0.159, respectively). Patients with HER2 amplification were more likely to have progressed post-TKI (p = 0.015). All five patients with HER2 amplification who progressed post-TKI also had EGFR sensitising mutations.
Table 3. Characteristics of Malaysian NSCLC patients with HER2 mutations and amplification compared with patients without the HER2 alterations.
Systematic review of HER2 mutations and amplification in NSCLC
Study selection
The database search performed on PubMed and Web of Science yielded 5,828 unique records for screening. Of these, 295 records were retrieved for full text screening; most records were excluded due to non-relevance, unsuitable article type (review articles and case reports), non-English abstract or article, unsuitable studies (in vitro or in vivo studies, and immunohistochemistry (IHC) results) or clinical trials performed before 2018. Upon applying the PICOS criteria, 94 articles were included; of these, 76 articles reported the prevalence of HER2 mutation and/or amplification, and 18 articles were prospective clinical trials evaluating the efficacy of treatment in NSCLC patients with HER2 mutations or amplification (Figure 2).
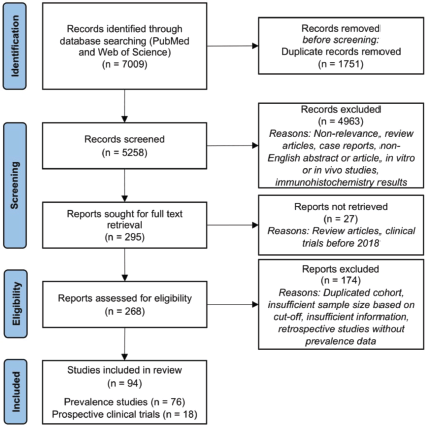
Figure 2. PRISMA diagram for inclusion of systematic review.
Prevalence of HER2 mutations and amplification
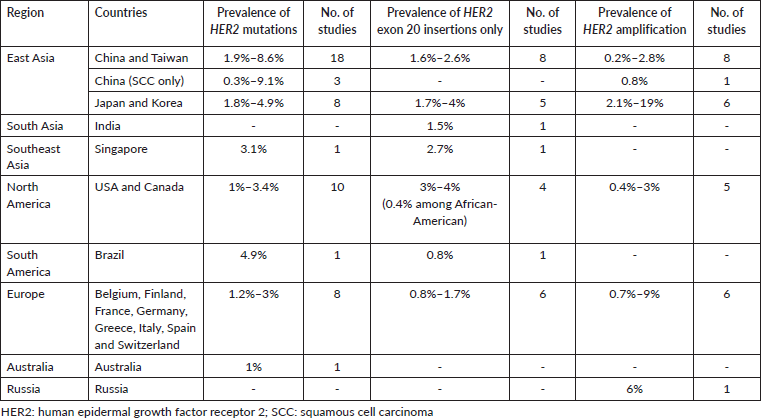
The prevalence of HER2 mutations and amplification reported in global studies are shown in Table 4. HER2 mutations in the studies were detected using various methods including Sanger sequencing, reverse transcription polymerase chain reaction (RT-PCR), NGS and matrix-assisted laser desorption ionisation-time of flight (MALDI-TOF) mass spectrometry (Supplementary Table S1 shows the full list of studies reporting the prevalence of HER2 mutations and amplification). The prevalence of HER2 mutations in NSCLC ranged from 0.3% to 9.1%. In some studies, the prevalence of HER2 exon 20 insertions were specifically reported, ranging from 0.4% among African-American populations in North America, to 4% in North America and East Asia. HER2 amplification was detected using fluorescent in situ hybridisation (FISH), silver in situ hybridisation (SISH), dual in situ hybridisation (DISH), multiplex ligation-dependent probe amplification (MLPA) or NGS. The prevalence of HER2 amplification varied widely, from 0.2% reported in China, up to 19% reported in Japan [17, 18]. Two studies which reported relatively high prevalence of HER2 amplification (14% and 19%) used the SISH or DISH method of detection [17, 19].
Efficacy of HER2-targeted therapies in NSCLC patients with HER2 mutations and amplification
Prospective clinical trials of various treatments for NSCLC patients with HER2 mutations and amplification are shown in Table 5.
Two phase II trials of afatinib in NSCLC patients with HER2 mutations were performed in post-progression patients; these trials revealed modest clinical benefits, i.e., ORR of 0%–7.7%, DCR of 53.9%–61.1%, median PFS of 2.8–4.0 months and median OS of 10–14 months. However, both studies did not compare the efficacy of afatinib in different HER2 exon 20 insertion variants [20, 21].
Poziotinib achieved a higher ORR (27.8%–27.9%), DCR (70%–73%) and PFS (5.5 months) compared to afatinib in patients on subsequent lines of therapy [22, 23]. Poziotinib’s DCR and PFS at subsequent lines of therapy were comparable to its use in the first line setting (ORR of 41%, DCR of 73% and PFS of 5.6 months) [22–24]. However, poziotinib did not receive United States Food and Drug Administration approval due to its modest efficacy, yet significant gastrointestinal and dermal toxicities [25].
Table 4. Prevalence of HER2 mutations and amplification in NSCLC reported in global studies.
Treatment of lung cancer patients with trastuzumab emtansine at various lines of therapy yielded an overall ORR of 38.1%–51.0%, DCR of 52.4%–83.3% and PFS 2.8–5.0 months [8, 11, 26]. Li et al [8] reported comparable responses to trastuzumab emtansine among patients when stratified according to HER2 status (mutation, amplification or combination of both). Although these trials recruited patients with central nervous system (CNS) metastasis, no subgroup analysis data were presented.
Pyrotinib has been investigated as a monotherapy, and in combination with apatinib for patients with HER2 mutation or amplification [27–31]. The ORR, DCR and PFS were generally lower with pyrotinib monotherapy at 19.2%–30.0%, 74.4%–85.0% and 5.6–6.9 months, respectively, compared to 35.7%–51.5%, 93.9%–100% and 6.9–8.0 months seen in pyrotinib + apatinib [27–31]. In a subgroup analysis of pyrotinib monotherapy, the ORR were comparable between patients with and without brain metastases (25.0% versus 31.3%).
Treatment of post-progression patients with trastuzumab deruxtecan (at a dose of either 5.4 or 6.4 mg/kg) in DESTINY-Lung01 and DESTINY-Lung02 yielded an encouraging ORR of 42.9%–54.9% and DCR of 90.4%–92.9% [12, 32]. In addition, trastuzumab deruxtecan at 6.4 mg/kg also yielded a more prolonged PFS of 8.2 months and OS of 18.6 months [12]. Trastuzumab deruxtecan seemed to achieve better treatment outcomes in post-progression lung cancer patients, compared to other HER2-targeted therapies (pyrotinib, afatinib, poziotinib and trastuzumab emtansine) (Table 5). In the DESTINY-Lung01 trial, comparable responses were observed with trastuzumab deruxtecan between patients with CNS metastasis and those without [12]. Safety-wise, although the DESTINY-Lung02 trial demonstrated similar efficacy of trastuzumab deruxtecan at both 5.4 and 6.4 mg/kg, a lower incidence of toxicities was observed with the 5.4 mg/kg dosage.
In the Drug Rediscovery Protocol (DRUP) trial, trastuzumab + pertuzumab demonstrated limited activity in patients with heavily pre-treated HER2-positive NSCLC (ORR 8.3%; DCR 38.0%; PFS 4.0 months; OS 10.0 months) [33]. Comparatively, trastuzumab + pertuzumab + docetaxel achieved improved outcomes in the IFCT 1703-R2D2 trial (ORR 29.0%; DCR 87.0%; PFS 4.0 months; OS 10.0 months) [34]. However, the IFCT 1703-R2D2 trial only included patients with stage III disease, while the DRUP trial recruited patients with metastatic disease.
Table 5. Summary of phase II clinical trials of HER2-targeted therapies for NSCLC patients with HER2 mutations and amplification.
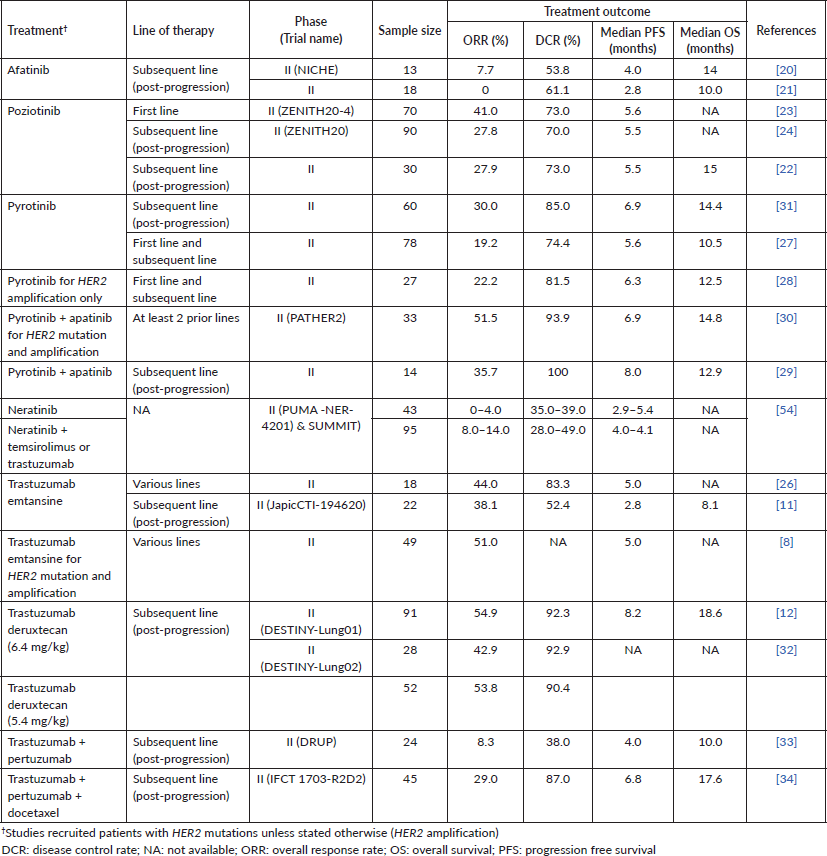
Finally, neratinib as monotherapy as well as in combination with temsirolimus or trastuzumab in NSCLC patients with HER2 alteration produced inferior ORR (0%–14%) and DCR (28%–49%) compared with poziotinib, pyrotinib, trastuzumab emtansine and trastuzumab deruxtecan.
Discussion
This article aims to elucidate the prevalence of HER2 mutations and amplification in NSCLC, as well as the clinical characteristics and mutational profiles of patients with these alterations, based on retrospective analysis of diagnostic NGS performed at a referral center in Malaysia. To the best of our knowledge, this article reports the first known statistics on HER2 alterations among lung cancer patients in Malaysia. We also performed a systematic literature review to summarise the available evidence on the prevalence of HER2 alteration in NSCLC and the treatment outcomes in these patients.
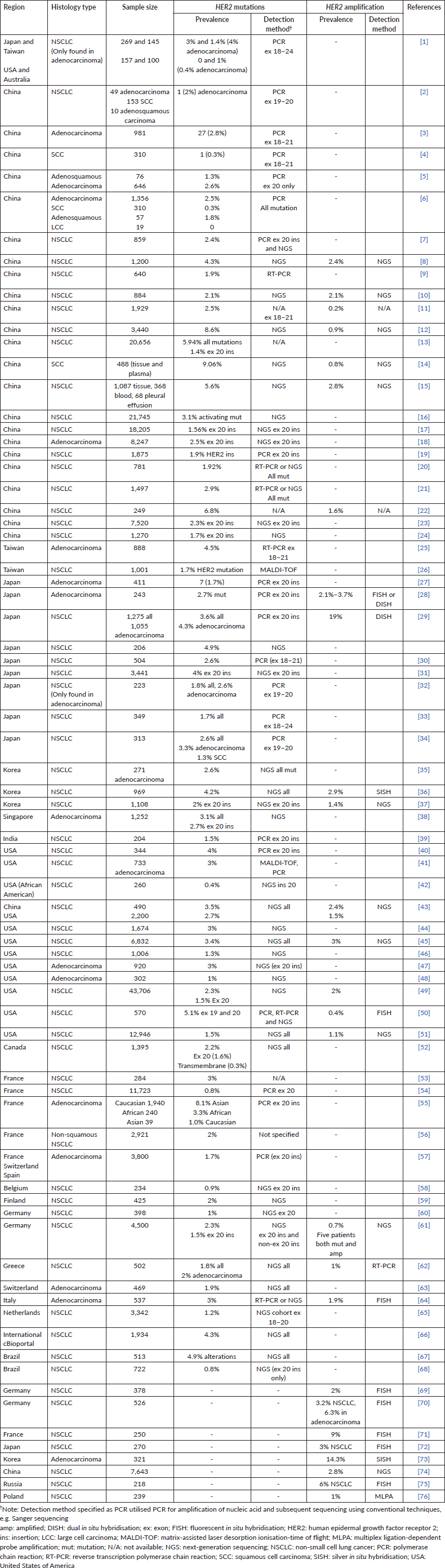
It is important to note that the frequency of HER2 alterations may vary depending on the detection modalities used, target region of test assay, tumour heterogeneity, NSCLC subtype and sample type. Our systematic review on the prevalence of HER2 mutations and amplification in NSCLC was analysed from a total of 76 articles; most articles described studies originated from East Asia, North America or Europe, with variations in the testing method used. The prevalence of HER2 mutations reported may be higher in studies using NGS for testing, as more variants can be detected using this modality. In contrast, Sanger sequencing demonstrates lower sensitivity compared to NGS or RT-PCR. The assessment of HER2 amplifications can be carried out utilising techniques such as FISH, SISH, DISH or NGS but currently, there is no standardised criteria for determining HER2 amplification in NSCLC [1]. Finally, HER2 expression can be evaluated using IHC. The current testing recommendation is to include HER2 mutation testing upfront as part of broad molecular profiling for NSCLC patients with advanced or metastatic disease, in particular, if approved therapies are available [1, 35].
In our retrospective analysis, HER2 alteration was seen in 5.8% of Malaysian NSCLC patients. Of these, 3.9% had HER2 mutations only and 1.5% had HER2 amplifications only, and a small subset (0.4%) of our patient cohort were both HER2-mutated and amplified. Our prevalence findings fall within the range reported in global studies (0.3%–9.1% for HER2 mutation and 0.2%–19% for HER2 amplification) (Table 4). Specifically, the prevalence of HER2 mutations (4.3%) was within the range reported by studies from East Asia (Table 4), with marginally lower overall prevalence from North America and Europe. The prevalence in this study was also slightly above Singapore (3.1%), the other Southeast Asian country with available published data. HER2 exon 20 insertions were the most common HER2 variants in our patient cohort. Similarly, available literature reported that most HER2 mutations (90%) occur in the form of HER2 exon 20 insertions, with Y772_A775dup (also referred to as A775_G776insYVMA, E770_A771insAYVM or A771_M774dup in scientific literature) being the most common subtype [3, 36]. Furthermore, in our patient cohort, HER2 exon 20 insertions were mostly mutually exclusive to other driver mutations, with only the S310S/Y mutation found in the extracellular ligand binding domain co-occurring with EGFR sensitising mutations. This finding is also mirrored in another retrospective study, which found only eight patients (out of 12946 NSCLC patients) who had both EGFR and HER2 mutations; of these eight patients, six patients had sensitising EGFR mutations and exon eight HER2 mutation (S310F/Y) [37]. However, it is unclear whether if a concurrent HER2 S310X mutation will affect response to EGFR TKIs.
In our patient cohort, those with HER2 mutations tend to be younger than non-HER2-mutants (median age 61 versus 64 years old; p = 0.046) and were inclined to be female and never-smokers (not statistically significant; p = 0.111 and 0.204, respectively) (Table 3). HER2 mutations have been reported to be significantly associated with never-smokers, patients of Asian origin and female patients [38, 39]. There may be a higher prevalence of HER2 mutations in NSCLC patients from East Asia, although this could be attributed to the greater number of studies conducted in this region. HER2 amplifications, on the other hand, have also been described as a potential mechanism of acquired resistance to EGFR TKI, as FISH analysis has revealed that HER2 was amplified in 12% of tumours with acquired resistance, versus only 1% of untreated lung adenocarcinomas [40]. Other gene amplifications (e.g., EGFR and MET) are also known to act as resistance drivers against targeted therapy [41, 42]. These gene amplifications may occur de novo, or develop post-progression. In our patient cohort, all five patients with HER2 amplification who progressed post-TKI also had EGFR sensitising co-mutations. These patients likely developed HER2 amplification as acquired resistance to EGFR TKI. For these patients, therapies that target both EGFR and HER2 may confer clinical benefit [43]. Future studies of the Malaysian NSCLC patient cohort with HER2 alterations could benefit from analysis of treatment modalities and their impact on survival outcomes.
Our analysis of 18 prospective phase II clinical trials of various treatments for NSCLC patients with HER2 alterations revealed promising treatment outcomes with trastuzumab deruxtecan, trastuzumab emtansine, pyrotinib, pyrotinib + apatinib and trastuzumab + pertuzumab + docetaxel. Both HER2 mutation and amplification in lung cancer may be indicators of benefit with HER2-targeted therapy. HER2 mutations particularly in the extracellular domain or kinase domain, as well as amplification, lead to HER2 hyperactivation of downstream signalling cascades such as the PI3K and MAPK pathways [8]. Emerging therapeutic agents such as ADCs work by the selective binding of the monoclonal antibody component to the receptor’s extracellular domain, and delivery of the cytotoxic payload to arrest malignant cell growth. Anti-HER2 ADCs have generally demonstrated clinical activity in lung cancers with HER2-activating mutations, irrespective of the level of protein expression [8].
A phase II trial investigating treatment with trastuzumab emtansine in NSCLC characterised by HER2 overexpression or mutation was stopped early due to limited efficacy [44]. The authors noted that IHC 3+ or IHC 2+/FISH-positive tumours showed limited response to the investigational agent in the study [44]. In the phase II DESTINY-Lung01 study, trastuzumab deruxtecan was also evaluated in HER2-overexpressed metastatic NSCLC (IHC 2+ and 3+) at two dose levels: 6.4 and 5.4 mg/kg. The ORR was 26.5% and 34.1%, DCR was 69.4% and 78.0%, PFS was 5.7 and 6.7 months and OS was 12.4 and 11.2 months at 6.4 and 5.4 mg/kg, respectively. Both trastuzumab deruxtecan doses showed consistent antitumor activity in heavily pre-treated patients with HER2-overexpressed NSCLC [45]. This is in contrast with trastuzumab emtansine, which demonstrated limited efficacy in HER2-overexpressed NSCLC; DCR were only 7% and 30% in IHC 2+ and 3+ cohorts, respectively [7, 44]. Trastuzumab deruxtecan has an 8:4 8:1 chemotherapy drug-to-antibody ratio, compared with trastuzumab emtansine’s 3.5:1 chemotherapy drug-to-antibody ratio, which may explain the improved efficacy of trastuzumab deruxtecan [46]. Additionally, the membrane permeability of the cytotoxic payload of trastuzumab deruxtecan contributes to the bystander effect of inducing apoptosis in neighbouring tumour cells [12, 46]. Nonetheless, this higher drug-to-antibody ratio also leads to increased toxicities associated with trastuzumab deruxtecan treatment, in particular interstitial lung disease. Likewise, the DESTINY-Breast03 trial demonstrated that trastuzumab deruxtecan conferred better clinical benefit compared to trastuzumab emtansine in HER2-positive breast cancer [5]. The incidence of interstitial lung disease was reported to be higher in breast cancer patients treated with trastuzumab deruxtecan (15%) compared with trastuzumab emtansine (3%), although no grade 4/5 event was seen with either treatment [5].
The common HER2 mutation, HER2 Y772_A775dup / A775_G776insYVMA, was identified to confer increased resistance to afatinib and chemotherapy treatments in patients with NSCLC [47–49]. However, it is unclear if this resistance extends to treatment with other HER2 TKIs and ADCs. NSCLC patients with HER2 mutations have a higher incidence of brain metastases compared with patients with EGFR or KRAS mutations [50]. Moreover, HER2 exon 20 YVMA insertion is also associated with a higher lifetime incidence of brain metastasis in advanced NSCLC, compared to non-YVMA cases [51]. This higher propensity for brain metastasis might contribute to the challenges faced in achieving effective responses to afatinib and chemotherapy treatments due to poor penetration of the blood-brain barrier. In the phase II trials for NSCLC patients with HER2 mutations, sub-group analyses revealed comparable outcomes in patients with CNS metastasis who were treated with trastuzumab deruxtecan or pyrotinib. This finding is encouraging as it indicates that these treatment approaches could be effective in managing patients with CNS metastasis. TP53 is a common co-mutation that may also affect treatment efficacy. Co-mutations in the TP53 pathway have been shown to confer additional resistance to afatinib therapy in lung cancer [52]. In breast cancer, TP53-mutated patients tended to have a worse prognosis with anti-HER2 TKI treatment compared to TP53-wild-type patients [53]. Given the frequent occurrence of TP53 co-mutations in NSCLC patients, further investigation is warranted to better understand its implications for HER2-targeted therapies.
Conclusion
In conclusion, in this retrospective analysis of diagnostic NGS performed at a referral center in Malaysia, HER2 alteration was present in 5.8% of Malaysian NSCLC patients. Of these, 3.9% had HER2 mutation, 1.5% had HER2 amplification and 0.4% had both HER2 mutation and amplification. Most (79.7%) of Malaysian NSCLC patients with HER2 mutation had HER2 exon 20 insertions, with Y772_A775dup being the most frequent HER2 mutation variant. These findings fall within the range reported in global studies; the prevalence of HER2 mutations in NSCLC reported in global studies ranged from 0.3% to 9.1%, whereas the prevalence of HER2 amplification ranged from 0.2% to 19%. A systematic review of prospective phase II clinical trials of various treatments for NSCLC patients with HER2 alterations revealed promising treatment outcomes with trastuzumab deruxtecan, trastuzumab emtansine, pyrotinib, pyrotinib + apatinib and trastuzumab + pertuzumab + docetaxel. Malaysian NSCLC patients with HER2 alteration are anticipated to similarly benefit from the abovementioned HER2-targeted therapies.
Acknowledgments
This study received grant support from AstraZeneca; however, the funder had no role in the study’s design, analysis or manuscript drafting. Medical writing assistance for this article was provided by Mediconnexions Consulting Sdn Bhd. The authors also extend their gratitude to the SJMC laboratory staff for their valuable contributions to the NGS testing process.
Conflicts of interest
PR declares consultancies and receipt of speaker fees from AstraZeneca and Thermo Fisher, as well as research grants from AstraZeneca and Roche. NYY declares conference travel support from AstraZeneca. KP declares no conflict of interest regarding the publication of this article.
Funding
This study received grant support from AstraZeneca.
Author contributions
Conceptualisation, NYY and PR; Supervision, PR; Funding Acquisition, PR; Data Curation, NYY and KP; Formal Analysis, NYY; Writing – Original Draft Preparation, NYY and PR; Writing – Review & Editing, NYY, KP and PR.
Data availability
Data supporting the findings of this study are available from the corresponding author upon request.
References
1. Ren S, Wang J, and Ying J, et al (2022) Consensus for HER2 alterations testing in non-small-cell lung cancer ESMO Open 7(1) 100395 https://doi.org/10.1016/j.esmoop.2022.100395 PMID: 35149428 PMCID: 8844658
2. Bang YJ, Van Cutsem E, and Feyereislova A, et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial Lancet 376(9742) 687–697 https://doi.org/10.1016/S0140-6736(10)61121-X PMID: 20728210
3. Zeng J, Ma W, and Young RB, et al (2021) Targeting HER2 genomic alterations in non-small cell lung cancer J Nat Cancer Center 1(2) 58–73 https://doi.org/10.1016/j.jncc.2021.04.001
4. Gutierrez C and Schiff R (2011) HER2: biology, detection, and clinical implications Arch Pathol Lab Med 135(1) 55–62 https://doi.org/10.5858/2010-0454-RAR.1 PMID: 21204711 PMCID: 3242418
5. Hurvitz SA, Hegg R, and Chung WP, et al (2023) Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial Lancet 401(10371) 105–117 https://doi.org/10.1016/S0140-6736(22)02420-5
6. Gatzemeier U, Groth G, and Butts C, et al (2004) Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer Ann Oncol 15(1) 19–27 https://doi.org/10.1093/annonc/mdh031
7. Peters S, Stahel R, and Bubendorf L, et al (2019) Trastuzumab emtansine (T-DM1) in patients with previously treated HER2-overexpressing metastatic non-small cell lung cancer: efficacy, safety, and biomarkers Clin Cancer Res 25(1) 64–72 https://doi.org/10.1158/1078-0432.CCR-18-1590
8. Li BT, Michelini F, and Misale S, et al (2020) HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers Cancer Discov 10(5) 674–687 https://doi.org/10.1158/2159-8290.CD-20-0215 PMID: 32213539 PMCID: 7196485
9. Ettinger DS, Wood DE, and Aisner DL, et al (2023) NCCN guidelines® insights: non-small cell lung cancer, version 2.2023 J Natl Compr Canc Netw 21(4) 340–350 https://doi.org/10.6004/jnccn.2023.0020 PMID: 37015337
10. Riudavets M, Sullivan I, and Abdayem P, et al (2021) Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations ESMO Open 6(5) 100260 https://doi.org/10.1016/j.esmoop.2021.100260 PMID: 34479034 PMCID: 8414039
11. Iwama E, Zenke Y, and Sugawara S, et al (2022) Trastuzumab emtansine for patients with non-small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations Eur J Cancer 162 99–106 https://doi.org/10.1016/j.ejca.2021.11.021
12. Li BT, Smit EF, and Goto Y, et al (2022) Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer N Engl J Med 386(3) 241–251 https://doi.org/10.1056/NEJMoa2112431 PMCID: 9066448
13. Malaysia MoH (2018) Malaysian Study on Cancer Survival (Putrajaya: Malaysia MoH)
14. Rajadurai P, Yap NY, and Mohamed Yousoof SB, et al (2023) Mutational profiling of lung cancer using next generation sequencing: a Malaysian real-world clinical diagnostic experience J Mol Pathol 4(1) 31–43 https://doi.org/10.3390/jmp4010004
15. Gao J, Aksoy BA, and Dogrusoz U, et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal Sci Signal 6(269) pl1 https://doi.org/10.1126/scisignal.2004088 PMID: 23550210 PMCID: 4160307
16. Page MJ, McKenzie JE, and Bossuyt PM, et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews BMJ 372 n71 https://doi.org/10.1136/bmj.n71 PMID: 33782057 PMCID: 8005924
17. Suzuki M, Shiraishi K, and Yoshida A, et al (2015) HER2 gene mutations in non-small cell lung carcinomas: concurrence with Her2 gene amplification and Her2 protein expression and phosphorylation Lung Cancer 87(1) 14–22 https://doi.org/10.1016/j.lungcan.2014.10.014
18. Xue X, Asuquo I, and Hong L, et al (2020) Catalog of lung cancer gene mutations among Chinese patients Front Oncol 10 1251 https://doi.org/10.3389/fonc.2020.01251 PMID: 32850378 PMCID: 7417348
19. Kim EK, Kim KA, and Lee CY, et al (2017) The frequency and clinical impact of HER2 alterations in lung adenocarcinoma PLoS One 12(2) e0171280 https://doi.org/10.1371/journal.pone.0171280 PMID: 28146588 PMCID: 5287480
20. Dziadziuszko R, Smit EF, and Dafni U, et al (2019) Afatinib in NSCLC with HER2 mutations: results of the prospective, open-label phase II NICHE trial of European thoracic oncology platform (ETOP) J Thorac Oncol 14(6) 1086–1094 https://doi.org/10.1016/j.jtho.2019.02.017 PMID: 30825613
21. Fan Y, Chen J, and Zhou C, et al (2020) Afatinib in patients with advanced non-small cell lung cancer harboring HER2 mutations, previously treated with chemotherapy: a phase II trial Lung Cancer 147 209–213 https://doi.org/10.1016/j.lungcan.2020.07.017 PMID: 32738416
22. Elamin YY, Robichaux JP, and Carter BW, et al (2022) Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: results from a phase II trial J Clin Oncol 40(7) 702–709 https://doi.org/10.1200/JCO.21.01113 PMCID: 8887948
23. Le X, Cornelissen R, and Garassino M, et al (2022) Poziotinib in non-small-cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2 trial J Clin Oncol 40(7) 710–718 https://doi.org/10.1200/JCO.21.01323 PMCID: 8887939
24. Sun S, Prelaj A, and Baik C, et al (2022) 26MO efficacy and safety of poziotinib in treatment-naïve HER2 exon 20 insertion (ex20ins) mutated non-small cell lung cancer (NSCLC): ZENITH20-4 Ann Oncol 33 S13 https://doi.org/10.1016/j.annonc.2022.01.035
25. FDA Briefing Document (2022) Poziotinib [https://www.fda.gov/media/161676/download]
26. Li BT, Shen R, and Buonocore D, et al (2018) Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial J Clin Oncol 36(24) 2532–2537 https://doi.org/10.1200/JCO.2018.77.9777 PMID: 29989854 PMCID: 6366814
27. Song Z, Li Y, and Chen S, et al (2022) Efficacy and safety of pyrotinib in advanced lung adenocarcinoma with HER2 mutations: a multicenter, single-arm, phase II trial BMC Med 20(1) 42 https://doi.org/10.1186/s12916-022-02245-z PMID: 35101045 PMCID: 8805254
28. Song Z, Lv D, and Chen SQ, et al (2022) Pyrotinib in patients with HER2-amplified advanced non-small cell lung cancer: a prospective, multicenter, single-arm trial Clin Cancer Res 28(3) 461–467 https://doi.org/10.1158/1078-0432.CCR-21-2936
29. Yang G, Xu H, and Xu F, et al (2021) P86.02 Pyrotinib combined with apatinib for HER2-mutant non-small cell lung cancer: interim analysis from a phase II clinical study J Thorac Oncol 16(3) S672–S673
30. Yang G, Xu H, and Yang Y, et al (2022) Pyrotinib combined with apatinib for targeting metastatic non-small cell lung cancer with HER2 alterations: a prospective, open-label, single-arm phase 2 study (PATHER2) BMC Med 20(1) 277 https://doi.org/10.1186/s12916-022-02470-6 PMID: 36031613 PMCID: 9422117
31. Zhou C, Li X, and Wang Q, et al (2020) Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase II study J Clin Oncol 38(24) 2753–2761 https://doi.org/10.1200/JCO.20.00297 PMID: 32614698
32. Goto K, Sang-We K, and Kubo T, et al (2022) LBA55 trastuzumab deruxtecan (T-DXd) in patients (Pts) with HER2-mutant metastatic non-small cell lung cancer (NSCLC): interim results from the phase 2 DESTINY-Lung02 trial Ann Oncol 33 Supp 7, S1422 https://doi.org/10.1016/j.annonc.2022.08.057
33. van Berge Henegouwen JM, Jebbink M, and Hoes LR, et al (2022) Trastuzumab and pertuzumab combination therapy for advanced pre-treated HER2 exon 20-mutated non-small cell lung cancer Eur J Cancer 171 114–123 https://doi.org/10.1016/j.ejca.2022.05.009 PMID: 35716537
34. Mazieres J, Lafitte C, and Ricordel C, et al (2022) Combination of trastuzumab, pertuzumab, and docetaxel in patients with advanced non–small-cell lung cancer harboring HER2 mutations: results from the IFCT-1703 R2D2 trial J Clin Oncol 40(7) 719–728 https://doi.org/10.1200/JCO.21.01455 PMID: 35073148
35. Rajadurai P, Cheah PL, and How SH, et al (2019) Molecular testing for advanced non-small cell lung cancer in Malaysia: consensus statement from the College of Pathologists, Academy of Medicine Malaysia, the Malaysian Thoracic Society, and the Malaysian Oncological Society Lung Cancer 136 65–73 https://doi.org/10.1016/j.lungcan.2019.08.005 PMID: 31446227
36. Brazel D, Kroening G, and Nagasaka M (2022) Non-small cell lung cancer with EGFR or HER2 exon 20 insertion mutations: diagnosis and treatment options BioDrugs 36(6) 717–729 https://doi.org/10.1007/s40259-022-00556-4 PMID: 36255589 PMCID: 9649507
37. Nagasaka M, Singh V, and Baca Y, et al (2022) The effects of HER2 alterations in EGFR mutant non-small cell lung cancer Clin Lung Cancer 23(1) 52–59 https://doi.org/10.1016/j.cllc.2021.08.012
38. Shigematsu H, Takahashi T, and Nomura M, et al (2005) Somatic mutations of the HER2 kinase domain in lung adenocarcinomas Cancer Res 65(5) 1642–1646 https://doi.org/10.1158/0008-5472.CAN-04-4235 PMID: 15753357
39. Sholl LM, Aisner DL, and Varella-Garcia M, et al (2015) Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience J Thorac Oncol 10(5) 768–777 https://doi.org/10.1097/JTO.0000000000000516 PMID: 25738220 PMCID: 4410843
40. Takezawa K, Pirazzoli V, and Arcila ME, et al (2012) HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation Cancer Discov 2(10) 922–933 https://doi.org/10.1158/2159-8290.CD-12-0108 PMID: 22956644 PMCID: 3473100
41. Coleman N, Hong L, and Zhang J, et al (2021) Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer ESMO Open 6(6) 100319 https://doi.org/10.1016/j.esmoop.2021.100319 PMID: 34837746 PMCID: 8637467
42. Yang H, Wen L, and Zhao C, et al (2022) EGFR amplification is a putative resistance mechanism for NSCLC-LM patients with TKI therapy and is associated with poor outcome Front Oncol 12 902664 https://doi.org/10.3389/fonc.2022.902664 PMID: 35978803 PMCID: 9376465
43. Gan J, Huang Y, and Liao J, et al (2021) HER2 amplification in advanced NSCLC patients after progression on EGFR-TKI and clinical response to EGFR-TKI plus pyrotinib combination therapy Onco Targets Ther 14 5297–5307 https://doi.org/10.2147/OTT.S335217 PMID: 34824536 PMCID: 8609241
44. Hotta K, Aoe K, and Kozuki T, et al (2018) A phase II study of trastuzumab emtansine in HER2-positive non-small cell lung cancer J Thorac Oncol 13(2) 273–279 https://doi.org/10.1016/j.jtho.2017.10.032 PMID: 29313813
45. Smit EF, Felip E, and Uprety D, et al (2022) 975P Trastuzumab deruxtecan in patients (pts) with HER2-overexpressing (HER2-OE) metastatic non-small cell lung cancer (NSCLC): results from the DESTINY-Lung01 trial Ann Oncol 33 S994–S995 https://doi.org/10.1016/j.annonc.2022.07.1103
46. Nader-Marta et al (2023) Antibody–drug conjugates: the evolving field of targeted chemotherapy for breast cancer treatment https://doi.org/10.1177/17588359231183679
47. Yang G, Xu H, and Hu J, et al (2022) Specific HER2 exon 20 Gly776 deletion-insertions in non-small cell lung cancer: structural analysis and sensitivity to HER2-targeted tyrosine kinase inhibitors Front Pharmacol 13 806737 https://doi.org/10.3389/fphar.2022.806737 PMID: 35330827 PMCID: 8940162
48. Fang W, Zhao S, and Liang Y, et al (2020) Mutation variants and co-mutations as genomic modifiers of response to afatinib in HER2-mutant lung adenocarcinoma Oncologist 25(3) e545–e554 https://doi.org/10.1634/theoncologist.2019-0547 PMID: 32162827 PMCID: 7066719
49. Liu Z, Wu L, and Cao J, et al (2018) Clinical characterization of ERBB2 exon 20 insertions and heterogeneity of outcomes responding to afatinib in Chinese lung cancer patients Onco Targets Ther 11 7323–7331 https://doi.org/10.2147/OTT.S173391 PMID: 30425522 PMCID: 6205822
50. Offin M, Feldman D, and Ni A, et al (2019) Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers Cancer 125(24) 4380–4387 https://doi.org/10.1002/cncr.32461 PMID: 31469421 PMCID: 6891113
51. Yang S, Wang Y, and Zhao C, et al (2021) Exon 20 YVMA insertion is associated with high incidence of brain metastasis and inferior outcome of chemotherapy in advanced non-small cell lung cancer patients with HER2 kinase domain mutations Transl Lung Cancer Res 10(2) 753–765 https://doi.org/10.21037/tlcr-20-559 PMID: 33718019 PMCID: 7947396
52. Yuan B, Zhao J, and Zhou C, et al (2020) Co-occurring alterations of ERBB2 exon 20 insertion in non-small cell lung cancer (NSCLC) and the potential indicator of response to afatinib Front Oncol 10 729 https://doi.org/10.3389/fonc.2020.00729 PMID: 32477948 PMCID: 7236802
53. Liu B, Yi Z, and Guan Y, et al (2022) Molecular landscape of TP53 mutations in breast cancer and their utility for predicting the response to HER-targeted therapy in HER2 amplification-positive and HER2 mutation-positive amplification-negative patients Cancer Med 11(14) 2767–2778 https://doi.org/10.1002/cam4.4652 PMID: 35393784 PMCID: 9302303
54. Li B, Gandhi L, and Besse B, et al (2021) FP14.15 neratinib-based combination therapy in HER2-mutant lung adenocarcinomas: findings from two international phase 2 studies J Thorac Oncol 16(3) S234 https://doi.org/10.1016/j.jtho.2021.01.158
Supplementary materials
Supplementary Table S1 provides the list of studies reporting the prevalence of HER2 mutations and amplification.
Supplementary Table S1. Studies reporting the prevalence of HER2 mutations and amplification.
References
1. Shigematsu H, Takahashi T, and Nomura M, et al (2005) Somatic mutations of the HER2 kinase domain in lung adenocarcinomas Cancer Res 65(5) 1642–1646 https://doi.org/10.1158/0008-5472.CAN-04-4235 PMID: 15753357
2. Feng S, Ling H, and Guo H, et al (2014) Infrequent ERBB2 mutations in Chinese patients with non-small cell lung cancer J Thorac Dis 6(5) 503–506 PMID: 24822110 PMCID: 4015026
3. Hu H, Pan Y, and Li Y, et al (2014) Oncogenic mutations are associated with histological subtypes but do not have an independent prognostic value in lung adenocarcinoma Onco Targets Ther 7 1423–1437 https://doi.org/10.2147/OTT.S58900 PMID: 25152623 PMCID: 4140237
4. Pan Y, Wang R, and Ye T, et al (2014) Comprehensive analysis of oncogenic mutations in lung squamous cell carcinoma with minor glandular component Chest 145(3) 473–479 https://doi.org/10.1378/chest.12-2679
5. Wang R, Pan Y, and Li C, et al (2014) Analysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomas J Thorac Oncol 9(6) 760–768 https://doi.org/10.1097/JTO.0b013e3182a406d1 PMID: 24481316
6. Wang R, Zhang Y, and Pan Y, et al (2015) Comprehensive investigation of oncogenic driver mutations in Chinese non-small cell lung cancer patients Oncotarget 6(33) 34300–34308 https://doi.org/10.18632/oncotarget.5549 PMID: 26486077 PMCID: 4741453
7. Song Z, Yu X, and Shi Z, et al (2016) HER2 mutations in Chinese patients with non-small cell lung cancer Oncotarget 7(47) 78152–78158 https://doi.org/10.18632/oncotarget.11313 PMID: 27825109 PMCID: 5363651
8. Wen S, Dai L, and Wang L, et al (2019) Genomic signature of driver genes identified by target next-generation sequencing in Chinese non-small cell lung cancer Oncologist 24(11) e1070–e1081 https://doi.org/10.1634/theoncologist.2018-0572 PMID: 30902917 PMCID: 6853120
9. Yang S, Song Z, and Cheng G (2019) Genomic alterations and survival in young patients aged under 40 years with completely resected non-small cell lung cancer Ann Transl Med 7(7) 140 https://doi.org/10.21037/atm.2019.03.39 PMID: 31157261 PMCID: 6511547
10. Li D, Ding L, and Ran W, et al (2020) Status of 10 targeted genes of non-small cell lung cancer in eastern China: a study of 884 patients based on NGS in a single institution Thorac Cancer 11(9) 2580–2589 https://doi.org/10.1111/1759-7714.13577 PMID: 32729257 PMCID: 7471050
11. Xue X, Asuquo I, and Hong L, et al (2020) Catalog of lung cancer gene mutations among chinese patients Front Oncol 10 1251 https://doi.org/10.3389/fonc.2020.01251 PMID: 32850378 PMCID: 7417348
12. Sun S, Du W, and Sun Q, et al (2021) Driver gene alterations profiling of Chinese non-small cell lung cancer and the effects of co-occurring alterations on immunotherapy Cancer Med 10(20) 7360–7372 https://doi.org/10.1002/cam4.4178 PMID: 34599863 PMCID: 8525092
13. Wang D, Yuan H, and Zhu H (2021) The characteristics of ERBB2 exon 20 insertion in a large cohort of Chinese NSCLC patients Cancer Res 81(13_Suppl)
14. Chen Y, Kong W, and Yu Z, et al (2022) Genetic alteration and PD-L1 expression profiles of Chinese patients with lung squamous cell carcinoma Pathol Res Pract 231 153761 https://doi.org/10.1016/j.prp.2022.153761 PMID: 35151031
15. Li H, Li X, and Lan S, et al (2022) ERBB2 mutation landscape in non-small cell lung cancer patients in Northeast China Tumori J 109(3) 276–281 https://doi.org/10.1177/03008916221101426
16. Fan Xu, Qingshan Li, and Wenxin LI, et al (2022) Molecular characteristics of ERBB2-activating mutations in Chinese patients with NSCLC J Clin Oncol 40(16) 8546 https://doi.org/10.1200/JCO.2022.40.16_suppl.8546
17. Yang L, Huang T, and Qu Y, et al (2022) Real-world frequency of non-small cell lung cancer with ERBB2 exon 20 insertion (Exon20ins) mutations by site of insertion J Clin Oncol 40(16) e15026 https://doi.org/10.1200/JCO.2022.40.16_suppl.e15026
18. Tian P, Zeng H, and Ji L, et al (2021) Lung adenocarcinoma with ERBB2 exon 20 insertions: comutations and immunogenomic features related to chemoimmunotherapy Lung Cancer 160 50–58 https://doi.org/10.1016/j.lungcan.2021.07.014 PMID: 34403912
19. Bu S, Wang R, and Pan Y, et al (2017) Clinicopathologic characteristics of patients with HER2 insertions in non-small cell lung cancer Ann Surg Oncol 24(1) 291–297 https://doi.org/10.1245/s10434-015-5044-8
20. Xu C, Wang W, and Zhuang W, et al (2017) P1.02-046 mutational subtypes and prognosis of non-small-cell lung cancer harboring HER2 mutations J Thorac Oncol 12(11) S1940 https://doi.org/10.1016/j.jtho.2017.09.777
21. Zhou J, Ding N, and Xu X, et al (2020) Clinical outcomes of patients with HER2-mutant advanced lung cancer: chemotherapies versus HER2-directed therapies Ther Adv Med Oncol 12 1758835920936090 https://doi.org/10.1177/1758835920936090 PMID: 32647540 PMCID: 7325548
22. Diao WY, Ding CL, and Yuan BY, et al (2021) Clinical characteristics and prognosis of HER2 gene phenotype in patients with non-small cell lung cancer Int J Gen Med 14 9153–9161 https://doi.org/10.2147/IJGM.S328908 PMID: 34880654 PMCID: 8646112
23. Liu Z, Wu L, and Cao J, et al (2018) Clinical characterization of ERBB2 exon 20 insertions and heterogeneity of outcomes to afatinib in Chinese lung cancers J Thorac Oncol 13(12) S1046–S1047 https://doi.org/10.1016/j.jtho.2018.10.017
24. Chen K, Pan G, and Cheng G, et al (2021) Immune microenvironment features and efficacy of PD-1/PD-L1 blockade in non-small cell lung cancer patients with EGFR or HER2 exon 20 insertions Thorac Cancer 12(2) 218–226 https://doi.org/10.1111/1759-7714.13748 PMCID: 7812071
25. Gow CH, Chang HT, and Lim CK, et al (2017) Comparable clinical outcomes in patients with HER2-mutant and EGFR-mutant lung adenocarcinomas Genes Chromosomes Cancer 56(5) 373–381 https://doi.org/10.1002/gcc.22442 PMID: 28063177
26. Chu CH, Huang YH, and Lee PH, et al (2022) Various impacts of driver mutations on the PD-L1 expression of NSCLC PLoS One 17(8) e0273207 https://doi.org/10.1371/journal.pone.0273207 PMID: 35980949 PMCID: 9387808
27. Serizawa M, Koh Y, and Kenmotsu H, et al (2014) Assessment of mutational profile of Japanese lung adenocarcinoma patients by multitarget assays Cancer 120(10) 1471–1481 https://doi.org/10.1002/cncr.28604 PMID: 24700479
28. Yoshizawa A, Sumiyoshi S, and Sonobe M, et al (2014) HER2 status in lung adenocarcinoma: a comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations Lung Cancer 85(3) 373–378 https://doi.org/10.1016/j.lungcan.2014.06.007 PMID: 25047676
29. Suzuki M, Shiraishi K, and Yoshida A, et al (2015) HER2 gene mutations in non-small cell lung carcinomas: concurrence with HER2 gene amplification and HER2 protein expression and phosphorylation Lung Cancer 87(1) 14–22 https://doi.org/10.1016/j.lungcan.2014.10.014
30. Tomizawa K, Suda K, and Onozato R, et al (2011) Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers Lung Cancer 74(1) 139–144 https://doi.org/10.1016/j.lungcan.2011.01.014 PMID: 21353324
31. Udagawa H, Matsumoto S, and Ohe Y, et al (2019) Clinical outcome of non-small cell lung cancer with EGFR/HER2 exon 20 insertions identified in the LC-SCRUM-Japan J Thorac Oncol 14(10) S224 https://doi.org/10.1016/j.jtho.2019.08.443
32. Sonobe M, Manabe T, and Wada H, et al (2006) Lung adenocarcinoma harboring mutations in the ERBB2 kinase domain J Mol Diagn 8(3) 351–356 https://doi.org/10.2353/jmoldx.2006.050132 PMID: 16825508 PMCID: 1867605
33. Yokoyama T, Kondo M, and Goto Y, et al (2006) EGFR point mutation in non-small cell lung cancer is occasionally accompanied by a second mutation or amplification Cancer Sci 97(8) 753–759 https://doi.org/10.1111/j.1349-7006.2006.00233.x PMID: 16863509 PMCID: 11158750
34. Takahashi T, Sonobe M, and Kobayashi M, et al (2010) Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene Ann Surg Oncol 17(3) 889–897 https://doi.org/10.1245/s10434-009-0808-7 PMID: 20183914
35. Li S, Choi YL, and Gong Z et al (2016) Comprehensive characterization of oncogenic drivers in Asian lung adenocarcinoma J Thorac Oncol 11(12) 2129–2140 https://doi.org/10.1016/j.jtho.2016.08.142 PMID: 27615396
36. Lee Y, Lee B, and Choi YL, et al (2022) Clinicopathologic and molecular characteristics in ERBB2 (HER2)-altered non-small cell lung cancer Virchows Archiv 481(SUPPL 1) S151–S152
37. Lee K, Jung HA, and Sun JM, et al (2020) Clinical characteristics and outcomes of non-small cell lung cancer patients with HER2 alterations in Korea Cancer Res Treat 52(1) 292–300 https://doi.org/10.4143/crt.2019.186 PMCID: 6962476
38. Tan AC, Saw SPL, and Chen J, et al (2022) Clinical and genomic features of HER2 exon 20 insertion mutations and characterization of HER2 expression by immunohistochemistry in East Asian non-small-cell lung cancer JCO Precis Oncol 6 e2200278 https://doi.org/10.1200/PO.22.00278 PMID: 36240473
39. Bhaumik S, Ahmad F, and Das BR (2016) Somatic mutation analysis of KRAS, BRAF, HER2 and PTEN in EGFR mutation-negative non-small cell lung carcinoma: determination of frequency, distribution pattern and identification of novel deletion in HER2 gene from Indian patients Med Oncol 33(10) 117 https://doi.org/10.1007/s12032-016-0828-7 PMID: 27637917
40. Cardarella S, Ortiz TM, and Joshi VA, et al (2012) The introduction of systematic genomic testing for patients with non-small-cell lung cancer J Thorac Oncol 7(12) 1767–1774 https://doi.org/10.1097/JTO.0b013e3182745bcb PMID: 23154547 PMCID: 3500523
41. Kris MG, Johnson BE, and Berry LD, et al (2014) Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs J Am Med Assoc 311(19) 1998–2006 https://doi.org/10.1001/jama.2014.3741
42. Araujo LH, Lammers PE, and Matthews-Smith V, et al (2015) Somatic mutation spectrum of non-small-cell lung cancer in African Americans: a pooled analysis J Thorac Oncol 10(10) 1430–1436 https://doi.org/10.1097/JTO.0000000000000650 PMID: 26301800 PMCID: 4618391
43. Xu C, Zhang Y, and Liu D, et al (2018) P1.01-99 detecting HER2 alterations by next generation sequencing (NGS) in patients with advanced NSCLC from the United States and China J Thorac Oncol 13(10) S502 https://doi.org/10.1016/j.jtho.2018.08.655
44. Sacher AG, Dahlberg SE, and Heng J, et al (2016) Association between younger age and targetable genomic alterations and prognosis in non-small-cell lung cancer JAMA Oncol 2(3) 313–320 https://doi.org/10.1001/jamaoncol.2015.4482 PMID: 26720421 PMCID: 4819418
45. Suh JH, Johnson A, and Albacker L, et al (2016) Comprehensive genomic profiling facilitates implementation of the National Comprehensive Cancer Network guidelines for lung cancer biomarker testing and identifies patients who may benefit from enrollment in mechanism-driven clinical trials Oncologist 21(6) 684–691 https://doi.org/10.1634/theoncologist.2016-0030 PMID: 27151654 PMCID: 4912374
46. Illei PB, Belchis D, and Tseng LH, et al (2017) Clinical mutational profiling of 1006 lung cancers by next generation sequencing Oncotarget 8(57) 96684–96696 https://doi.org/10.18632/oncotarget.18042 PMID: 29228562 PMCID: 5722514
47. Pillai RN, Behera M, and Berry LD, et al (2017) HER2 mutations in lung adenocarcinomas: a report from the lung cancer mutation consortium Cancer 123(21) 4099–4105 https://doi.org/10.1002/cncr.30869 PMID: 28743157 PMCID: 5650517
48. Miller TE, Yang M, and Bajor D, et al (2018) Clinical utility of reflex testing using focused next-generation sequencing for management of patients with advanced lung adenocarcinoma J Clin Pathol 71(12) 1108–1115 https://doi.org/10.1136/jclinpath-2018-205396 PMID: 30228211 PMCID: 6900927
49. Ou SHI, Madison R, and Robichaux JP, et al (2019) Characterization of 648 non-small cell lung cancer (NSCLC) cases with 28 unique HER2 exon 20 insertions J Clin Oncol 37(15) 9063 https://doi.org/10.1200/JCO.2019.37.15_suppl.9063
50. Patil T, Mushtaq R, and Marsh S, et al (2020) Clinicopathologic characteristics, treatment outcomes, and acquired resistance patterns of atypical EGFR mutations and HER2 alterations in Stage IV non-small-cell lung cancer Clin Lung Cancer 21(3) E191–E204 https://doi.org/10.1016/j.cllc.2019.11.008
51. Nagasaka M, Singh V, and Baca Y, et al (2022) The effects of HER2 alterations in EGFR mutant non-small cell lung cancer Clin Lung Cancer 23(1) 52–59 https://doi.org/10.1016/j.cllc.2021.08.012
52. Kuang S, Fung AS, and Perdrizet KA, et al (2022) Upfront next generation sequencing in non-small cell lung cancer Curr Oncol 29(7) 4428–4437 https://doi.org/10.3390/curroncol29070352 PMID: 35877212 PMCID: 9319994
53. Couraud S, Souquet PJ, and Paris C, et al (2015) BioCAST/IFCT-1002: epidemiological and molecular features of lung cancer in never-smokers Eur Respir J 45(5) 1403–1414 https://doi.org/10.1183/09031936.00097214 PMID: 25657019
54. Barlesi F, Souquet J, and Paris C, et al (2016) Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet 387(10026) 1415–1426 https://doi.org/10.1016/S0140-6736(16)00004-0 PMID: 26777916
55. Saffroy R, Morère JF, and Bosselut N, et al (2017) Impact of country of birth on genetic testing of metastatic lung adenocarcinomas in France: African women exhibit a mutational spectrum more similar to Asians than to Caucasians Oncotarget 8(31) 50792–50803 https://doi.org/10.18632/oncotarget.15132 PMID: 28881604 PMCID: 5584205
56. Lamy T, Cabarrou B, and Planchard D, et al (2021) Biomarker testing in older patients treated for an advanced or metastatic non-squamous non-small-cell lung cancer: the French ESME real-life multicenter cohort experience Cancers (Basel) 14(1) 92 https://doi.org/10.3390/cancers14010092
57. Mazières J, Peters S, and Lepage B, et al (2013) Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives J Clin Oncol 31(16) 1997–U307 https://doi.org/10.1200/JCO.2012.45.6095 PMID: 23610105
58. D’Haene N, Le Mercier M, and De Neve N, et al (2015) Clinical application of targeted next-generation sequencing for lung cancer patients: a Belgian experience Cancer Res 75(22) A1–39 https://doi.org/10.1158/1538-7445.TRANSCAGEN-A1-39
59. Mäki-Nevala S, Sarhadi VK, and Rönty M, et al (2016) Hot spot mutations in Finnish non-small cell lung cancers Lung Cancer 99 102–110 https://doi.org/10.1016/j.lungcan.2016.06.024 PMID: 27565922
60. Tafe LJ, Pierce KJ, and Peterson JD, et al (2016) Clinical genotyping of non-small cell lung cancers using targeted next-generation sequencing: utility of identifying rare and co-mutations in oncogenic driver genes Neoplasia 18(9) 577–583 https://doi.org/10.1016/j.neo.2016.07.010 PMID: 27659017 PMCID: 5031899
61. Volckmar AL, Christopoulos P, and Kirchner M, et al (2021) Targeting rare and non-canonical driver variants in NSCLC - an uncharted clinical field Lung Cancer 154 131–141 https://doi.org/10.1016/j.lungcan.2021.02.022 PMID: 33667718
62. Tsoulos N, Papadopoulou E, and Metaxa-Mariatou V, et al (2017) Tumor molecular profiling of NSCLC patients using next generation sequencing Oncol Rep 38(6) 3419–3429 PMID: 29130105 PMCID: 5783588
63. Grosse A, Grosse C, and Rechsteiner M, et al (2019) Analysis of the frequency of oncogenic driver mutations and correlation with clinicopathological characteristics in patients with lung adenocarcinoma from Northeastern Switzerland Diagn Pathol 14(1) 18 https://doi.org/10.1186/s13000-019-0789-1
64. Dall’Olio FG, Conci N, and Rossi G, et al (2020) Comparison of sequential testing and next generation sequencing in advanced lung adenocarcinoma patients - a single centre experience Lung Cancer 149 5–9 https://doi.org/10.1016/j.lungcan.2020.08.008 PMID: 32932213
65. Steeghs EMP, Groen HJM, and Schuuring E, et al (2022) Mutation-tailored treatment selection in non-small cell lung cancer patients in daily clinical practice Lung Cancer 167 87–97 https://doi.org/10.1016/j.lungcan.2022.04.001 PMID: 35461050
66. Wei X, Gao X, and Zhang X, et al (2019) P2.14-49 Molecular characteristics of HER2 mutations in non-small cell lung cancer J Thorac Oncol 14(10) S848–S849 https://doi.org/10.1016/j.jtho.2019.08.1834
67. Mascarenhas E, Gelatti AC, and Araújo LH, et al (2021) Comprehensive genomic profiling of Brazilian non-small cell lung cancer patients (GBOT 0118/LACOG0418) Thorac Cancer 12(5) 580–587 https://doi.org/10.1111/1759-7714.13777 PMCID: 7919136
68. de Oliveira Cavagna R, Zaniolo BG, and de Paula FE, et al (2022) ERBB2 exon 20 insertions are rare in Brazilian non-small cell lung cancer Thorac Cancer 13(23) 3402–3407 https://doi.org/10.1111/1759-7714.14605 PMID: 36251951 PMCID: 9715798
69. Heinmöller P, Gross C, and Beyser K, et al (2003) HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin Clin Cancer Res 9(14) 5238–5244 PMID: 14614004
70. Grob TJ, Kannengiesser I, and Tsourlakis MC, et al (2012) Heterogeneity of ERBB2 amplification in adenocarcinoma, squamous cell carcinoma and large cell undifferentiated carcinoma of the lung Modern Pathol 25(12) 1566–1573 https://doi.org/10.1038/modpathol.2012.125
71. Tagliamento M, Auclin E, and Valent A, et al (2022) 1090P HER2 copy number variation in non-small cell lung cancer (NSCLC) Ann Oncol 33(7) S1050 https://doi.org/10.1016/j.annonc.2022.07.1215
72. Inoue Y, Matsuura S, and Kurabe N, et al (2015) Clinicopathological and survival analysis of japanese patients with resected non-small-cell lung cancer harboring NKX2-1, SETDB1, MET, HER2, SOX2, FGFR1, or PIK3CA gene amplification J Thorac Oncol 10(11) 1590–1600 https://doi.org/10.1097/JTO.0000000000000685 PMID: 26536195
73. Kim EK, Kim KA, and Lee CY, et al (2017) The frequency and clinical impact of HER2 alterations in lung adenocarcinoma Plos One 12(2) e0171280 https://doi.org/10.1371/journal.pone.0171280 PMID: 28146588 PMCID: 5287480
74. Zhou C, Ai X, and Gu D, et al (2021) P53.07 clinical and genomic insights into of Chinese lung cancer patients with HER2 amplification J Thorac Oncol 16(10) S1128 https://doi.org/10.1016/j.jtho.2021.08.556
75. Kobyakov DS, Avdalyan AM, and Klimachev VV, et al (2015) Non-small cell lung cancer: HER2 oncogene status Arkh Patol 77(2) 3–9 https://doi.org/10.17116/patol20157723-9 PMID: 26027392
76. Lewandowska MA, Czubak K, and Klonowska K, et al (2015) The use of a two-tiered testing strategy for the simultaneous detection of small EGFR mutations and EGFR amplification in lung cancer Plos One 10(2) e0117983 https://doi.org/10.1371/journal.pone.0117983 PMID: 25719557 PMCID: 4342230






