Head and neck cancer burden in India: an analysis from published data of 37 population-based cancer registries
Sonali Bagal1,a, Atul Budukh1,2,b, Jarnail Singh Thakur3,c, Tapas Dora4, Burhanuddin Qayyumi5, Divya Khanna6,d, Dolorosa Fernandes7, Priyal Chakravarti1,e, Ravikant Singh5, Suvarna Patil8, Rajesh Dikshit1,2,f and Pankaj Chaturvedi1,2,g
1Centre for Cancer Epidemiology (CCE), Advance Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre (TMC), Navi Mumbai 410210, India
2Homi Bhabha National Institute (HBNI), Training School Complex, Anushakti Nagar, Mumbai 400094, India
3Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh 160012, India
4Homi Bhabha Cancer Hospital (HBCH), Tata Memorial Centre (TMC), Sangrur 148001, India
5Homi Bhabha Cancer Hospital and Research Centre (HBCH&RC), Tata Memorial Centre (TMC), Muzaffarpur 842004, India
6Mahamana Pandit Madan Mohan Malaviya Cancer Centre (MPMMCC), Tata Memorial Centre (TMC), Varanasi 221005, India 7Homi Bhabha Cancer Hospital and Research Centre (HBCH&RC), Tata Memorial Centre (TMC), Visakhapatnam 530053, India
8Bhaktshreshtha Kamalakarpant Laxman Walawalkar (BKLW) Hospital, Diagnostic and Research Centre, Ratnagiri 415606, India
ahttps://orcid.org/0000-0002-2510-1751
bhttps://orcid.org/0000-0001-6723-802X
chttps://orcid.org/0000-0002-8270-4268
dhttps://orcid.org/0000-0001-7856-8059
ehttps://orcid.org/0000-0003-2163-796X
fhttps://orcid.org/0000-0003-4830-0486
ghttps://orcid.org/0000-0002-3520-1342
Abstract
Head and neck cancer (HNC) is a major public health problem in India. This article presents the HNC burden in different regions of India. The published population-based cancer registries (PBCRs) data from the National Cancer Registry Programme, Bengaluru, and the Tata Memorial Centre, Mumbai, India, were utilised. The 37 PBCRs were divided into six regions including central, east, north, northeast, west and south. The age-standardised incidence rate of HNC was 25.9 (95% CI 25.7–26.1) and 8.0 (95% CI 7.9–8.1) per 100,000 population, respectively, in males and females. HNC accounted for about 26% of all cancer cases in males and 8% in females. The risk of developing HNC was 1 in 33 for males and 1 in 107 for females. The northeastern registries reported the highest incidence rate 31.7 per 100,000 population in males followed by northern (28.5), central (28.3), western (24.4), southern (23.9) and eastern (18.3). In females, the incidence was in the range of 6.2–10.1 per 100,000 population. For all PBCRs together, the HNC burden was two to three times higher in the age group 60+ as compared to 20–39 years. The HNC burden in India is higher than in the USA, UK, Australia, Africa and Brazil. The PBCRs from the south-east Asia region such as the Colombo district, Sri Lanka, as well as Siraha, Saptari, Dhanusha and Mohattari – Nepal have also reported a high burden of HNC. All regions reported mouth as a leading cancer site followed by tongue, larynx, hypopharynx and tonsil except the northeastern region registries where hypopharynx was the top leading cancer. The burden of other sites of HNC is low. Raising awareness of the disease and associated risk factors, providing early detection services, as well as easy access to diagnosis and treatment are required. The government should focus on building the infrastructure and capacity building to control this disease.
Keywords: head and neck cancer, mouth neoplasm, oropharyngeal neoplasms, hypopharyngeal neoplasm, laryngeal neoplasms, incidence, registry, India
Correspondence to: Atul Budukh
Email: atul.budukh@gmail.com and budukham@tmc.gov.in
Published: 21/09/2023
Received: 10/07/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In India, the number of cancer cases is rising. According to GLOBOCAN 2020, there will be 2.1 million new cancer cases in India by 2040, an increase of 57.5% from the year 2020 [1–3]. Moreover, one in nine Indians has a lifetime risk of developing cancer [3]. Head and neck cancer (HNC), in particular, accounts for 30% of the all-cancer cases [4]. Furthermore, a significant rise in the incidence of HNC was noted in the Indian population-based cancer registries (PBCRs) of Aurangabad, Delhi, Chennai, and Bhopal for males, and Nagpur for females [5]. HNC includes the cancers of the oral cavity, pharynx, and larynx – primarily originating from the mucosal epithelium, but also from less common sites like salivary glands, and nose, sinuses, muscles and nerves [6]. Squamous cell carcinoma makes up most cases of HNC [7].
In India, more than 65% of patients with HNC attend the hospital with locally advanced disease [8–10]. Late-stage presentation, lack of access to cancer care and failure to complete treatment lead to poor survival in HNC patients [11, 12]. High-quality data from PBCRs are required to address this public health issue as well as to make better infrastructure and policy-related decisions about disease prevention. However, in India, PBCRs cover less than 15% of the population [3, 13, 14]. Several challenges have been reported in establishing PBCRs in resource-constrained countries like India [15, 16].
This research paper aims to summarise the region-wise incidence of HNC based on the published data of the registries of that region. The study utilised the published and publicly available Indian PBCRs data. Data from 28 PBCRs available in public domain of the National Cancer Registry Programme (NCRP), Bengaluru [5] and 9 PBCRs established by the Tata Memorial Centre (TMC), Mumbai (for the states of Maharashtra, Punjab, Uttar Pradesh, Bihar, and Andhra Pradesh as well as the Chandigarh-Union Territory) were included [17–25]. The Barshi registry was the country’s first rural cancer registry, established by TMC, Mumbai in 1987 and regularly submitted data to NCRP.
There is a variation in the definition of HNC by the National Cancer Institute and the International Agency for Research on Cancer (IARC) [26]. Therefore, there is a need to specify the anatomical subsites covered in this study. Here, we have defined HNC based on the International Classification of Diseases (ICD-10) topography codes which include lip (C00), tongue (C01-02), mouth (C03-06), tonsil (C09), other oropharynx (C10), nasopharynx (C11), hypopharynx (C12-13), pharynx unspecified (C14) and larynx (C32). The cancers of salivary gland, maxillary sinus and nasal cavity are not included in the present data due to the different etiology of the disease.
Method
As per the geographical area, the 37 PBCRs were categorised into six regions including central, eastern, northern, northeastern, southern and western India. The central region included Bhopal, Nagpur and Wardha PBCRs (year 2012–2016). The eastern region included Kolkata PBCR (year 2012–2015). The northern region included Chandigarh, Delhi, Mansa, Patiala, Sangrur, SAS Nagar, Varanasi and Muzaffarpur PBCRs (year 2012–2019). Cachar, Dibrugarh, Kamrup urban, Manipur state, Meghalaya, Mizoram state, Nagaland, Pasighat and Sikkim state, Tripura state and West Arunachal PBCRs are included under the northeast region (year 2012–2016). The southern region included Bengaluru, Chennai, Hyderabad, Kollam, Thiruvananthapuram and Visakhapatnam PBCRs (year 2012–2018). The western region included Ahmedabad urban, Aurangabad, Barshi rural, Mumbai, Osmanabad-Beed, Pune, Ratnagiri and Sindhudurg (year 2012–2018). The registry data available in the public domain of NCRP are for the year 2012–2016 except for Bhopal, Kolkata, Mumbai, Osmanabad-Beed (2012–2015), Delhi, Bangalore (2012–2014) and Hyderabad registries (2014–2016). The TMC PBCRs included data for the period 2017–2018 of Chandigarh, Sangrur, SAS Nagar, Mansa, Visakhapatnam, Ratnagiri and Sindhudurg cancer registries. Data for Varanasi and Muzaffarpur PBCRs were for the years 2018–2019 and 2018, respectively. The number of cancer cases and the population of the respective age group (as per age group 0–4, 5–9, …, 70–74, 75+) were entered into Microsoft Excel for each registry. The region-wise number of cancer cases registered was combined, and the population covered by registries of the respective region was merged. The entered data of registries merged region-wise for calculation. The age-standardised incidence rate (ASIR) was calculated using the statistical methods defined by the IARC [27]. For calculating the HNC burden, we have considered the period year 2012–2019, though the data collected are from different periods. The calculations are based on the data available in the particular period. We have taken the period 2012–2019 which is inclusive of different periods from the data reported by the respective registries. As cancer is a chronic disease, its pattern and burden will not change in a short period.
All defined incidents HNC (age group 0–75+) of each registry were taken as numerators, while the estimated population of geographic area from each PBCR for the respective period was taken as the denominator. The analysis has been done using published data year for both numerator and denominator. As per the published data, the total cases with unknown age group were less than 0.2% in the HNC sites. The HNC cases with unknown ages were equally distributed in all the age groups. For calculating the ASIR, first, the age-specific rates for the age groups were calculated by using the number of cases in that age group as the numerator and the population of that respective age group as the denominator. Later, the ASIR per 100,000 population was calculated using the world standard population along with a 95% confidence interval (CI) [27]. The cumulative risk was calculated using the statistical methods defined by the IARC [27] to know the risk that an individual would have of developing cancer in a certain age span if no other causes of death had occurred.
Results
The incidence of HNC in India
The all-site cancer incidence rate was 103.7 and 102.4 per 100,000 population for males and females, respectively. The ASIR for HNC was 25.9 (95% CI 25.7–26.1) and 8.0 (95% CI 7.9–8.1) per 100,000 population for males and females, respectively. The burden of HNC as compared to all-site cancer is mentioned in Figure 1.
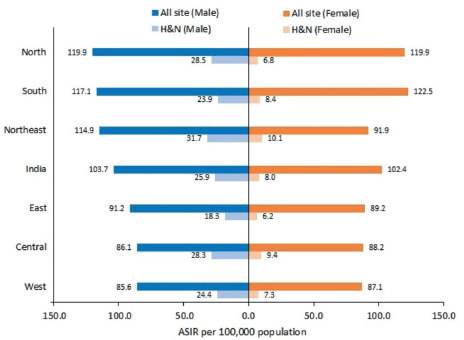
Figure 1. Comparison of all site cancer burden with HNC burden in different regions of India (2012–2019).
The HNC burden across different age groups and gender
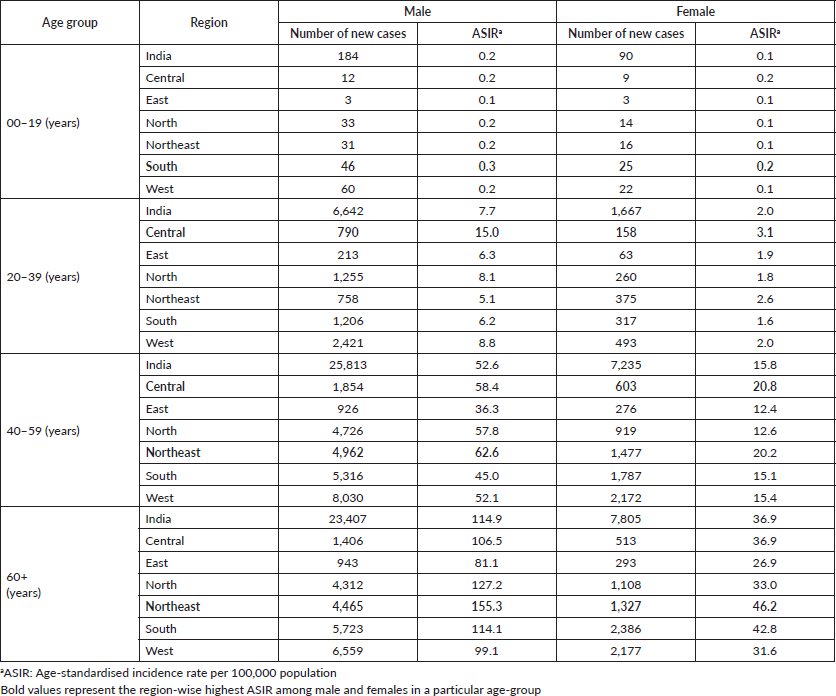
We observed that ASIR for HNC was increasing with age. The HNC burden was the lowest in the age group 0–19 years (0.1–0.3 in males and 0.1–0.2 in females per 100,000 population). For the age group 20–39 years, males residing in the central region had the highest burden of HNC (15.0 per 100,000 population).
For the age group 40–59 years, the northeastern registries reported the highest burden of the disease among males with ASIR 62.6 per 100,000 population; and among females, it was high in the central region with ASIR 20.8 per 100,000 population. The incidence rate of age group 40–59 was in the range of 36.3–62.6 and 12.4–20.8 per 100,000 population in males and females, respectively.
Compared to all other age groups, age 60 and above reported higher ASIR for both males and females. For all registries combined, the overall ASIR for males and females (age 60+ years) was 114.9 and 36.9 per 100,000 population, respectively. In the age group 60+, the highest incidence was reported by registries from the northeastern region for both males and females 155.3 and 46.2 per 100,000 population. The region-wise HNC burden among different age groups is presented in Table 1.
Table 1. HNC burden among different age groups by region and gender (2012–2019).
The HNC incidence in different regions
The northeastern registries have reported the highest incidence of HNC in males 31.7 (95% CI 31.1–32.3) per 100,000 population followed by the registries from northern and central India, 28.5 (95% CI 27.9–29.1) and 28.3 (95% CI 27.4–29.2) per 100,000 population respectively. For females, the HNC ASIR among different regions was in the range of 6.2–10.1 per 100,0000 population. The burden of HNC as compared to all-site cancer is mentioned in Figure 2.
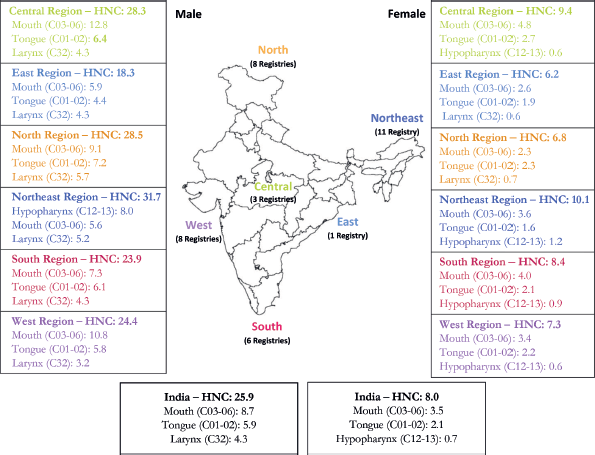
Figure 2. HNC burden along with the first three leading associated sites (2012–2019).
Proportion of HNC to all-site cancer in different regions of India and other countries
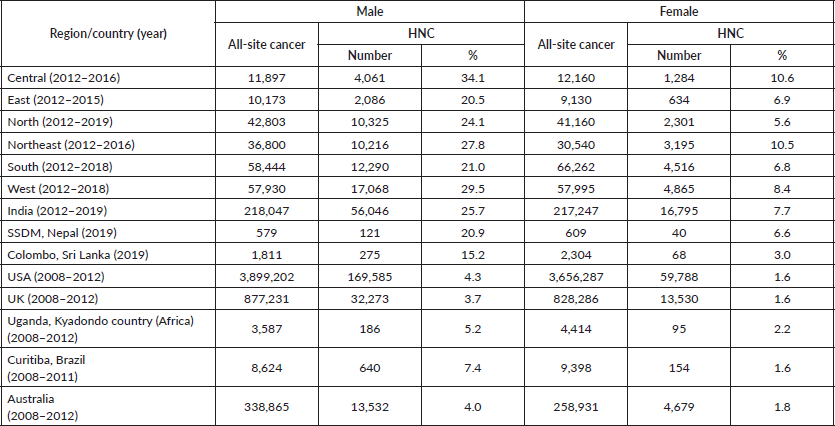
As per data analysis of all the registries, among the 218,047 registered cancers in males, 56,046 (25.7%) cases were HNC; while among 217,247 registered cancers in females, 16,795 (7.7%) cases were HNC. Among males, the highest proportion of HNC was reported in the central region (34.1%) followed by western (29.5%), northeastern (27.8%), northern (24.1%), southern (21.0%) and eastern (20.5%) regions. Females had a lower proportion of HNC. Among females, the highest proportion was reported in the central region (10.6%) followed by the northeastern (10.5%), western (8.4%), eastern (6.9%), southern (6.8%) and northern (5.6%) regions.
In other parts of the world, especially developed countries such as USA, the UK and Australia, the proportion of HNC to all-site cancer is much lower than in India; 4.3%, 3.7% and 4.0%, respectively for males while for females the proportion is less than 2%. Moreover, the proportion of HNC to all-site cancer in developing nations including Siraha, Saptari, Dhanusha and Mohattari (SSDM) – Nepal, Columbo-Sri Lanka, Uganda-Kyadondo country (Africa) and Curitiba-Brazil is lower than India for both males and females [28–30]. The proportion of HNC as compared to all-site cancer by regions in India as well as other countries is mentioned in Table 2.
Leading sites in HNC in different regions of India
Among males, mouth, tongue, larynx, hypopharynx and tonsil were the top five leading cancer sites in the central, eastern, northern, southern and western regions PBCRs while PBCRs in the northeastern region reported hypopharynx as the leading cancer site followed by mouth, larynx tongue and tonsil.
The mouth was the commonest site for HNC for males (ASIR ranges from 5.6 to 12.8 per 100,000 population) and for females (ASIR ranges from 2.3 to 4.8 per 100,000 population). The tongue cancer ASIR per 100,000 population was in the range of 4.4–7.2 and 1.6–2.7 for males and females, respectively.
Table 2. Proportion of HNC to all-site cancer by regions of India along with other countries by gender.
The site-wise HNC burden with 95% CI for both genders is presented in Table 3. A comparison of the first three associated sites of HNC rate among different regions of India is presented in Figures 2.
Table 3. Region and gender-wise ASIR of leading HNC sites (2012–2019).
Gender-wise cumulative risk in different regions
In India, one in 33 males and one in 107 females are at risk of developing HNC. The highest cumulative risk was reported among the northeastern region male population (1 in 26 was at risk of developing HNC). This was followed by northern (1 in 30), central (1 in 31), southern (1 in 35), western (1 in 36) and eastern (1 in 47). For other anatomical sites, the cumulative risk was high for mouth cancer followed by tongue.
Region-wise, females from the northeastern region showed the highest cumulative risk (1 in 83), followed by central (1 in 92), southern (1 in 101), western (1 in 118), northern (1 in 128) and eastern (1 in 141). For other anatomical sites, similar to the male population, mouth and tongue cancer cumulative risk was the highest. The cumulative risk for both genders is presented in Table 3.
Comparison of associated sites of HNC between India and other countries
Overall, India shows higher ASIR for mouth, tongue and hypopharynx cancers compared to the USA, UK, Australia, Uganda-Kyadondo country (Africa) and Brazil as well as SSDM – Nepal and Colombo – Sri Lanka for both genders [28–30].
Mouth cancer ASIR for the Indian male population was 8.7 per 100,000 population which was more or less similar to Columbo – Sri Lanka and SSDM-Nepal, 8.2 and 6.2 per 100,000 population, respectively. The ASIR was much lower for the USA (1.8), Australia (2.1) and UK (2.2) compared to India. For females, the mouth cancer ASIR is 3.5 in India; while it ranges from 0.8 to 1.7 in other countries.
Compared to India where tongue cancer ASIR was reported 5.9 per 100,000 male population, the ASIR for other countries ranged from 1.2 to 4.1 per 100,000 population. For females, the ASIR for tongue cancer ranged from the lowest at 0.6 in Uganda-Kyadondo country (Africa) to the highest in India at 2.1 per 100,000 population. The hypopharynx cancer ASIR for males ranged the lowest from 0.5 in Uganda-Kyadondo country (Africa) to the highest in India 3.1 per 100,000 population; whereas for females, it was the highest in India (0.7) to lowest in SSDM-Nepal and Australia (0.1).
There is a variation in ASIR for other HNC sites. The ASIR for laryngeal cancer was reported highest in Brazil (5.8) followed by India and the USA (4.3). The ASIR for cancer of the tonsils was higher in developed nations including the USA (2.6), Australia (2.1) and UK (1.9). Cancer of the nasopharynx was reported highest for both males and females in Uganda, Kyadondo country (Africa) 2.7 and 1.8 per 100,000 population, respectively. Cancer of the lip was reported highest in Australia for both males (4.4) and females (1.3) compared to other countries. The associated site-wise comparison of the HNC burden of India with other countries is illustrated in Figure 3.
Discussion
The present paper describes the burden of HNC in the different regions of India, based on the published data of 37 PBCRs. As per GLOBOCAN 2020, HNC accounts for 28% of total cancer cases in males and 7% of cases in females in India [1]. Our study found an almost similar proportion of HNC with 26% males and 8% females. In India, the risk of developing HNC is noteworthy in males as well as in females. However, compared to females, males are three times higher in terms of HNC incidence. With regard to age, the incidence of HNC was higher among the age group 40 and above. This finding reflects the need to direct health interventions to address this issue in these age groups. Furthermore, there are limitations in the cancer mortality data in India. It has been reported that insufficient or inaccurate cause-of-death certification and the lack of all-cause mortality data are the reasons behind some registries' low death certificate-only percentages [8]. Not all PBCRs have their mortality data available in the public domain. Hence, we have not analysed the same in this study. However, as per GLOBOCAN 2020, the age-standardised mortality rate of HNC in India for males and females is 14.2 and 4.1 per 100,000 population, respectively [1].
The highest HNC incidence was observed in the northeastern region of India for both males and females. Moreover, when compared to other regions, the incidence of hypopharynx cancer was the highest for both males and females in the northeastern region. The high prevalence of smoking and smokeless forms of tobacco use in the northeast region could be one of the reasons [31].
Compared to other countries such as the USA, UK, Australia, Africa and Brazil overall HNC burden is higher in India. The cancer registries from south-east Asia region such as the Colombo – Sri Lanka as well as SSDM – Nepal have also reported a high burden of HNC [29, 30]. The HNC burden may be higher than the reported data as several registries have reported under-reporting of the cases.
There are several risk factors associated with HNC. The use of tobacco, reverse smoking and alcohol consumption, are the major risk factors for HNC. In addition, poor oral hygiene, micronutrient deficiencies, and human papillomavirus (HPV) infection are reported risk factors for HNC [4, 26, 32]. The variation in ASIR for different HNC sites may be due to risk factor exposure. Mouth and tongue cancer cases are higher in India due to higher tobacco use specifically smokeless form [33]. The regions of India such as central, eastern, northern, southern and western have reported that mouth cancer was the leading cancer site in males followed by tongue, larynx and hypopharynx. Furthermore, the National Family Health Survey-5 also reported that tobacco prevalence is comparatively high in Uttar Pradesh, Bihar, Arunachal Pradesh, Manipur, Meghalaya, Mizoram, Nagaland, Sikkim, Tripura and West Bengal states; it is in the range of 41%–73% in males and 5%–62% in females among these states [34]. Moreover, countries with similar settings such as Sri Lanka and Nepal also reported that higher intake of tobacco and alcohol use increases the risk of HNC [35, 36]. Regarding infectious diseases as a risk factor, a hospital-based study conducted in India reported that HPV prevalence was highest in the oropharynx (41.7%) followed by the hypopharynx (38.9%) and oral cavity (28.4%) [37]. Furthermore, it has been reported that Epstein–Barr virus (EBV) is associated with nasopharyngeal cancer [38]. Conversely, the associated risk factors of HNC in developed countries such as Australia were due to tobacco use especially smoked form as well as alcohol consumption [39]. Additionally, increased sun exposure may be a contributing factor in Australia's higher lip cancer incidence [40].
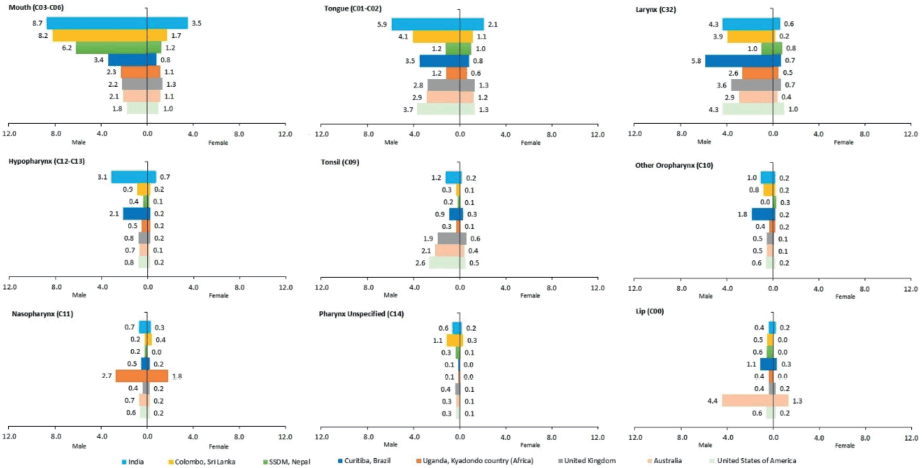
Figure 3. Site-wise comparison of the HNC burden of India with other countries.
The burden of associated sites of HNC such as tonsils, other oropharynx, nasopharynx, lip and pharynx unspecified is low in both genders. These cancer sites are considered rare HNC [41]. However, the nasopharyngeal cancer burden in some parts of the northeastern region was high which could be due to high consumption of smoked meat, and EBV infection [42, 43].
In this analysis, we have utilised the data of newly established PBCRs at Varanasi, Uttar Pradesh state and Muzaffarpur, Bihar state [21, 22]. These two states are the most populous states of India. Estimating the burden of the disease was difficult in the past because Bihar and Uttar Pradesh lacked PBCRs. We need to focus on the Uttar Pradesh state of India as the Varanasi registry has reported the highest burden of oral cancer (ASIR: 18.4 per 100,000 population) [21]. The prevalence of tobacco in these states is higher as compared to the national average [34]. The screening by oral visual examination for the high-risk population has resulted in a reduction in oral cancer mortality as per the randomised controlled trial conducted in the Indian population [44]. Furthermore, the cancer registry has played an important role in monitoring the screening programme [45].
Indian PBCRs survival studies have reported low survival in patients with oral cavity cancer [11] as compared to the United States and Europe [46, 47]. There is a dire need to develop and implement cost-effective strategies for cancer prevention and control through early detection and timely treatment. PBCRs will help in the monitoring of these activities. There is a pressing need to strengthen the tobacco control programme in India. The National Tobacco Quit Line services are available in different regions of India, offering cessation services in regional languages [48, 49]. Raising cancer and risk factor awareness in the community will support prevention activities. School education programmes are effective in raising cancer awareness in the community [50].
This study has provided the burden of HNC by utilising the registry data from different regions of India. The burden of HNC varies in different regions of India may be due to the number of PBCRs present in a particular region, coverage of cancer registration and access to diagnostic and treatment facilities. Establishing PBCRs has several challenges such as a lack of cooperation from the cancer-treating centres, inadequate medical records, social stigma about cancer and insufficient funding. To overcome these challenges, PBCR should develop strong institutional relationships and build rapport with data sources as well as interact with patients and their relatives for more accurate information about the disease as well as sociodemographic data [13]. Furthermore, the government authority should make cancer a notifiable disease for a smooth cancer registration process. The results have to be interpreted with caution as there may be underreporting of cases and few registries are recently established. The cancer incidence data for the year 2017–2018 from the PBCRs were not available in the public domain of NCRP at the time of manuscript preparation.
The government of India is playing an important role in strengthening the early detection services of oral, breast, and cervical cancer; however, there are several challenges in implementing these services in the community. Even though the national programme for noncommunicable diseases offers population-based screening, a study suggests low lifetime screening rates (4%–7% in Punjab and Haryana) which is due to logistics and training issues of health workers. Moreover, the uptake of oral cancer screening is extremely poor in both genders [51]. To address this significant public health issue, we must concentrate on finding effective ways to establish an early detection programme. It is reported that the involvement of trained community health workers in screening programme is feasible and effective method [52].
Prominent cancer centres across the nation should step up while assisting capacity building in order to help reduce the unmet needs for cancer control services; for instance, TMCs that are established in various geographical regions of India are playing a key role in developing infrastructure, and capacity building for cancer prevention activities to tackle this important public health problem.
The data generated through this study indicate that the HNC burden is high in India and these cancers should be tackled on a priority basis. As most of these cancers are preventable and the major associated risk factors are tobacco and alcohol consumption, it is recommended that the government should increase awareness of the disease and the harmful effects of tobacco and alcohol consumption among the population. Also, the public health department of the state and central governments should prioritise developing the infrastructure and capacity building in addition to offering early detection services, and facilitating quick access to diagnosis and treatment. Oral cancer screening programme needs to be strengthened. We recommend that medical practitioners as well as oral healthcare providers such as dentists should be trained in diagnosing oral premalignant disorders and oral cancer cases. There should be a hazel-free referral pathway in the healthcare system between healthcare providers and tertiary cancer centres.
Conclusion
1 in 33 males and 1 in 107 females are at risk of developing HNC in India. Mouth and tongue cancer are commonly prevalent HNC among both genders across India, except males in the northeastern region indicating hypopharynx as a leading cancer site. The burden of HNC is high, especially among the male population. Intensive awareness of risk factors such as tobacco, alcohol and HPV is required. Also, strengthening early detection services is required. Further research and policies on improving the uptake of available cancer screening are required for better cancer control and prevention.
List of abbreviations
ASIR, Age-standardised incidence rate; CI, Confidence interval; EBV, Epstein–Barr virus; HNC, Head and neck cancer; HPV, Human papillomavirus; IARC, International Agency for Research on Cancer; NCRP, National Cancer Registry Programme; PBCRs, Population-based cancer registries; TMC, Tata Memorial Centre.
Acknowledgment
We gratefully acknowledge Dr RA Badwe (Director, TMC, Mumbai, India) for his guidance. We also acknowledge Dr Satyajit Pradhan (Director, Mahamana Pandit Madan Mohan Malaviya Cancer Centre (MPMMCC), TMC, Varanasi, Uttar Pradesh, India), Dr Umesh Mahantshetty (Director, Homi Bhabha Cancer Hospital and Research Centre (HBCH&RC), TMC, Visakhapatnam, Andhra Pradesh, India) and Dr Ashish Gulia (Director, Homi Bhabha Cancer Hospital and Research Centre (HBCH&RC), TMC, Mullanpur, Punjab, India) for their valuable support to cancer registry. We appreciate and acknowledge NCRP, Bengaluru, India, for publicly available cancer registry data. We acknowledge all population-based cancer registry staff for their efforts in collecting the cancer data.
Author contribution
Sonali Bagal: Data collection, data analysis, writing – review and editing, quality control.
Atul Budukh: Conceptualisation, methodology, writing – original draft, supervision.
Jarnail Singh Thakur: Writing – review and editing.
Tapas Dora: Writing – review and editing, literature search.
Burhanuddin Qayyumi: Data analysis, writing – review and editing, literature search.
Divya Khanna: Data analysis, writing – review and editing, quality control.
Dolorosa Fernandes: Data analysis writing – review and editing, quality control.
Priyal Chakravarti: Literature search, assisted in reviewing and editing, quality control.
Ravikant Singh: Writing – review and editing.
Suvarna Patil: Writing – review and editing.
Rajesh Dikshit: Writing – review and editing.
Pankaj Chaturvedi: Writing – review and editing, quality control.
Conflicts of interest
The author(s) declare that they have no conflict of interest.
Funding
None.
References
1. Ferlay J, Ervik M, and Lam F, et al (2020) Global Cancer Observatory (Lyon: International Agency for Research on Cancer) https://gco.iarc.fr/ Date accessed: 07/06/23
2. World Health Organization (2020) WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All (Geneva: WHO)
3. Sathishkumar K, Chaturvedi M, and Das P, et al (2022) Cancer incidence estimates for 2022 & projection for 2025: result from National Cancer Registry Programme, India Indian J Med Res 156 598–607 PMID: 36510887 PMCID: 10231735
4. Kulkarni MR (2013) Head and neck cancer burden in India Int J Head Neck Surg 3(4) 29–35 https://doi.org/10.5005/jp-journals-10001-1132
5. ICMR National Centre for Disease Informatics and Research (2020) National Cancer Registry Programme Report Bengaluru, India (Bengaluru: ICMR National Centre for Disease Informatics and Research) https://ncdirindia.org/All_Reports/PBCR_Annexures/Default.aspx Date accessed: 07/06/23
6. National Cancer Institute (2018) Head and Neck Cancer (United states National Cancer Institute, National Institute of Health) https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet Date accessed: 07/06/23
7. Pindborg JJ, Reichart PA, and Smith CJ (1997). Histological Typing of Cancer and Precancer of the Oral Mucosa (Geneva: WHO) https://apps.who.int/iris/handle/10665/43552] Date accessed: 07/06/23
8. Mathur P, Sathishkumar K, and Chaturvedi M, et al (2020) Cancer statistics, 2020: report from National Cancer Registry Programme, India JCO Glob Oncol 6 1063–1075 https://doi.org/10.1200/GO.20.00122 PMID: 32673076 PMCID: 7392737
9. ICMR National Centre for Disease Informatics and Research (2021) Report of the Hospital Based Cancer Registries (Bengaluru: ICMR National Centre for Disease Informatics and Research) https://ncdirindia.org/All_Reports/HBCR_2021/ Date accessed: 07/06/23
10. Homi Bhabha Cancer Hospital (2018) Hospital Based Cancer Registry Annual Report (Sangrur: Homi Bhabha Cancer Hospital) https://tmc.gov.in/tmh/pdf/Reports/HBCR%20Sangrur%20Report-2018%2008-03-2021.pdf Date accessed: 07/06/23
11. Sankaranarayanan R and Swaminathan R (2011) Cancer Survival in Africa, Asia, the Caribbean and Central America (Lyon: International Agency for Research on Cancer, World Health Organization) https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Cancer-Survival-In-Africa-Asia-The-Caribbean-And-Central-America-2011 Date accessed: 07/06/23
12. Budukh AM, Chaudhary D, and Sancheti S, et al (2021) Determinants of completion of cancer directed treatment: an experience from a rural cancer centre, Sangrur, Punjab state, India Ecancermedicalscience 15 1313 https://doi.org/10.3332/ecancer.2021.1313
13. Bhatia A, Victora CG, and Beckfield J, et al (2021) “Registries are not only a tool for data collection, they are for action”: cancer registration and gaps in data for health equity in six population-based registries in India Int J Cancer 148 2171–2183 https://doi.org/10.1002/ijc.33391
14. Budukh A, Bagal S, and Thakur JS, et al (2023) Pediatric cancer burden in different regions of India: analysis of published data from 33 population-based cancer registries Indian Pediatr 60(7) 541–545 https://doi.org/10.1007/s13312-023-2931-0 PMID: 37078481
15. Parkin D and Sanghvi L (1991) Chapter 14: cancer registration in developing countries Cancer Registration: Principles and Methods (Lyon: IARC Scientific Publication) no 95 pp 185–198 https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Cancer-Registration-Principles-And-Methods-1991</a> Date accessed: 07/06/23
16. Budukh AM, Pradhan S, and Singh VB, et al (2023) Cancer pattern in Varanasi district from Uttar Pradesh state of India, a foundation for cancer control based on the first report of the population-based cancer registry Indian J Cancer [ahead of print] PMID: 36861723
17. Budukh A, Thakur JS, and Dikshit R, et al (2022) Cancer Incidence and Mortality in Sangrur District, Punjab, India: 2017-2018 Tata Memorial Centre (TMC), Mumbai and Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India https://tmc.gov.in/tmh/pdf/Reports/Sangrur%20PBCR%20Report%202017-2018.pdf Date accessed: 07/06/23
18. Budukh A, Thakur JS, and Dikshit R, et al (2022) Cancer Incidence and Mortality in Mansa District, Punjab, India: 2017-2018 Tata Memorial Centre (TMC), Mumbai and Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India https://tmc.gov.in/tmh/pdf/Reports/Mansa%20PBCR%20Report%202017-2018.pdf Date accessed: 07/06/23
19. Thakur JS, Budukh A, and Chaturvedi P, et al (2022) Cancer Incidence and Mortality in SAS Nagar District, Punjab, India: 2017-2018 Tata Memorial Centre (TMC), Mumbai and Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India https://tmc.gov.in/tmh/pdf/Reports/SAS%20Nagar%20PBCR%20Report%202017-2018.pdf Date accessed: 07/06/23
20. Thakur JS, Budukh A, and Chaturvedi P, et al (2022) Cancer Incidence and Mortality in Chandigarh Union Territory, India: 2017-2018 Tata Memorial Centre (TMC), Mumbai and Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India https://tmc.gov.in/tmh/pdf/Reports/Chandigarh%20PBCR%20Report%202017-2018 Date accessed: 07/06/23
21. Budukh A, Khanna D, and Bagal S, et al (2022) Cancer Incidence and Mortality in Varanasi District, Uttar Pradesh, India: 2018-2019 Tata Memorial Centre, Mumbai, India https://tmc.gov.in/tmh/pdf/Reports/Varanasi%20PBCR%20Detail%20report%20%202018-2019.pdf Date accessed: 07/06/23
22. Budukh A, Bagal S, and Pandey N, et al (2023) Cancer Incidence and Mortality in Muzaffarpur, Bihar State, India: 2018. Tata Memorial Centre (TMC), Mumbai, India https://tmc.gov.in/tmh/pdf/Reports/Muzaffarpur%20PBCR_2018.pdf Date accessed: 07/06/23
23. Lokhande D, Sarade M, and Bhise M, et al (2023) Cancer Incidence and Mortality in Sindhudurg District, Maharashtra State, India: 2017-2018 Tata Memorial Centre (TMC), Mumbai https://tmc.gov.in/tmh/PDF/Sindhudurg%20report%202017-2018_June%202023.pdf Date accessed: 07/06/23
24. Tata Memorial Centre (2023) Cancer Incidence and Mortality in Ratnagiri District, Maharashtra State, India: 2017-2018 (Mumbai: Tata Memorial Centre)
25. Tata Memorial Centre (2023) Cancer Incidence and Mortality in Visakhapatnam District, Andhra Pradesh, India: 2017-2018 (Mumbai: Tata Memorial Centre)
26. Gormley M, Creaney G, and Schache A, et al (2022) Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors Br Dent J 233 780–786 https://doi.org/10.1038/s41415-022-5166-x PMID: 36369568 PMCID: 9652141
27. Boyle P and Parkin D (1991) Chapter 11: statistical methods for registries Cancer Registration: Principles and Methods (Lyon: IARC Scientific Publication) no 95 pp 126–158 https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Cancer-Registration-Principles-And-Methods-1991 Date accessed: 07/06/23
28. Bray F, Colombet M, and Mery L, et al (2017) Cancer Incidence in Five Continents Vol. XI [Electronic Version] (Lyon: International Agency for Research on Cancer) https://ci5.iarc.fr Date accessed: 07/06/23
29. National Cancer Control Programme and Ministry of Health (2021) Cancer Incidence and Mortality Data Colombo District, Sri Lanka (Population-Based Cancer Registry): 2013-2019 (Colombo: National Cancer Control Programme, Ministry of Health) https://www.nccp.health.gov.lk/storage/post/pdfs/PCBR%20Books.pdf Date accessed: 07/06/23
30. Dhimal M, Dahal U, and Khadka K, et al (2022) Cancer Incidence and Mortality in Selected Districts of Nepal in 2019 (Kathmandu: Nepal Health Research Council) https://nhrc.gov.np/wp-content/uploads/2022/08/Population-Based-Cancer-Registry-Report-2019-1.pdf Date accessed: 07/06/23
31. Shanker N, Mathur P, and Das P, et al (2021) Cancer scenario in North-East India & need for an appropriate research agenda Indian J Med Res 154 27 https://doi.org/10.4103/ijmr.IJMR_347_20 PMID: 34782528 PMCID: 8715693
32. Johnson DE, Burtness B, and Leemans CR, et al (2020) Head and neck squamous cell carcinoma Nat Rev Dis Primers 6 92 https://doi.org/10.1038/s41572-020-00224-3 PMID: 33243986 PMCID: 7944998
33. Global Adult Tobacco Survey https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/global-adult-tobacco-survey Date accessed: 07/06/23
34. Government of India, Ministry of Health and Family Welfare National Family Health Survey (NFHS-5) 2019-21 http://rchiips.org/nfhs/factsheet_NFHS-5.shtml Date accessed: 07/06/23
35. Chang CP, Siwakoti B, and Sapkota A, et al (2020) Tobacco smoking, chewing habits, alcohol drinking and the risk of head and neck cancer in Nepal Int J Cancer 147 866–875 https://doi.org/10.1002/ijc.32823
36. Amarasinghe AAHK, Usgodaarachchi US, and Johnson NW, et al (2018) High prevalence of lifestyle factors attributable for oral cancer, and of oral potentially malignant disorders in rural Sri Lanka Asian Pac J Cancer Prev 19 2485–2492 PMID: 30256041 PMCID: 6249476
37. Gholap D, Mhatre S, and Chaturvedi P, et al (2022) Prevalence of human papillomavirus types in head and neck cancer sub-sites in the Indian population Ecancermedicalscience 16 1358 https://doi.org/10.3332/ecancer.2022.1358 PMID: 35510141 PMCID: 9023304
38. Dias JM, Santana IVV, and da Silva VD, et al (2022) Analysis of Epstein-Barr virus (EBV) and PD-L1 expression in nasopharyngeal carcinoma patients in a non-endemic region Int J Mol Sci 23(19) 11720 https://doi.org/10.3390/ijms231911720 PMID: 36233023 PMCID: 9569432
39. Australian Institute of Health and Welfare (2021) Cancer in Australia Cancer Series No. 133 Cat. No. CAN 144 (Canberra: AIHW)
40. NSW Cancer Registry Statistical Reporting (Cancer Institute NSW) https://www.cancer.nsw.gov.au/ Date accessed: 07/06/23
41. Mailankody S, Bajpai J, and Budukh A, et al (2023) Epidemiology of rare cancers in India and South Asian countries – remembering the forgotten Lancet Reg Health Southeast Asia 12 100168 https://doi.org/10.1016/j.lansea.2023.100168
42. Lourembam DS, Singh AR, and Sharma TD, et al (2015) Evaluation of risk factors for nasopharyngeal carcinoma in a high-risk area of India, the Northeastern Region Asian Pac J Cancer Prev 16 4927–4935 https://doi.org/10.7314/APJCP.2015.16.12.4927 PMID: 26163617
43. Saikia A, Raphael V, and Shunyu NB, et al (2016) Analysis of Epstein Barr virus encoded RNA expression in nasopharyngeal carcinoma in North-Eastern India: a chromogenic in situ hybridization based study Iran J Otorhinolaryngol 28 267–274 PMID: 27602338 PMCID: 4994986
44. Sankaranarayanan R, Ramadas K, and Thomas G, et al (2005) Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial Lancet 365 1927–1933 https://doi.org/10.1016/S0140-6736(05)66658-5 PMID: 15936419
45. Budukh AM, Dikshit R, and Chaturvedi P (2021) Outcome of the randomized control screening trials on oral, cervix and breast cancer from India and way forward in COVID-19 pandemic situation Int J Cancer 149 1619–1620 https://doi.org/10.1002/ijc.33712 PMID: 34152021 PMCID: 8427005
46. De Angelis R, Sant M, and Coleman MP, et al (2014) Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5 – a population-based study Lancet Oncol 15 23–34 https://doi.org/10.1016/S1470-2045(13)70546-1
47. SEER Cancer Statistics Review 1975-2009: National Cancer Institute https://seer.cancer.gov/archive/csr/1975_2009_pops09/results_merged/topic_survival.pdf Date accessed: 07/06/23
48. Budukh A, Chakravarti P, and Mulase D, et al (2020) Annual Report of Tobacco Quitline 2019 (Mumbai: Tata Memorial Centre)
49. Chethi Reddy BR, Musale D, and Kadam D, et al (2023) Challenges faced by tobacco quitline services during the COVID-19 pandemic J Substance Use 1–6 https://doi.org/10.1080/14659891.2023.2167744
50. Budukh A, Shah S, and Kulkarni S, et al (2022) Tobacco and cancer awareness program among school children in rural areas of Ratnagiri district of Maharashtra state in India Indian J Cancer 59 80–86
51. Patel KK, Chakravarti P, and Chaturvedi P, et al (2022) The fifth round of the National Family Health Survey of India 2019 to 2021 reported low screening uptake alarming to strengthen the implementation of early detection services of the cervix, breast and oral cancer Int J Cancer 150 1734–1736 https://doi.org/10.1002/ijc.33958 PMID: 35128640
52. Thampi V, Hariprasad R, and John A, et al (2022) Feasibility of training community health workers in the detection of oral cancer JAMA Netw Open 5 e2144022 https://doi.org/10.1001/jamanetworkopen.2021.44022 PMID: 35040966 PMCID: 8767429






