Clinical validation and diagnostic accuracy of 99mTc-EDDA/HYNIC-TOC compared to 111In-DTPA-octreotide in patients with neuroendocrine tumours: the LACOG 0214 study
Cristina M Moriguchi-Jeckel1,2, Rafael R Madke3, Graciane Radaelli1, Alice Viana3, Patrícia Nabinger3, Bruna Fernandes3, Gustavo Gössling4, Eduardo H Berdichevski5, Eduardo Vilas5, Juliana Giacomazzi4, Matheus Soares Rocha4, João Alfredo Borges6, Elias Hoffmann6,7, Samuel Greggio1,2, Gianina T Venturin1, Carlos H Barrios4, Facundo Zaffaroni4, Gustavo Werutsky4 and Jaderson C da Costa1
1Instituto do Cérebro do Rio Grande do Sul – Brain Institute (BraIns), Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Av Ipiranga, Porto Alegre 90619-900, Brazil
2Escola de Ciências da Saúde e da Vida (PUCRS), Av Ipiranga, Porto Alegre 90619-900, Brazil
3Grupo RPH, Av Ipiranga, Porto Alegre 90619-900, Brazil
4Latin American Cooperative Oncology Group (LACOG), Av Ipiranga, Porto Alegre 90619-900, Brazil
5Hospital São Lucas da PUCRS, Av Ipiranga, Porto Alegre 90610-001, Brazil
6P3DMED, Rua Gomes Jardim, 201 Sala 1109A, Porto Alegre 90620-130, Brazil
7Núcleo de Imagens Médicas (Nimed), P96A do Tecnopuc – PUCRS, Porto Alegre 90619-900, Brazil
Abstract
99mTc-EDDA/HYNIC-TOC is an easily available and cheaper radionuclide that could be used for somatostatin-receptor-based imaging of neuroendocrine tumours (NETs). We aimed to evaluate the diagnostic performance of 99mTc-EDDA/HYNIC-TOC compared to111In-DTPA-octreotide in patients (pts) with NETs. We performed a prospective diagnostic study including pts with biopsy-confirmed NET and at least one visible lesion at conventional imaging. Two independent nuclear medicine physicians evaluated pts who underwent 99mTc and 111In scans and images. The primary outcome was comparative diagnostic accuracy of 99mTc and 111In. Secondary outcomes include safety.
Nine pts were included and performed 14 paired scans. Overall, 126 lesions were identified. 99mTc demonstrated superior sensitivity both when all images were analysed (93.7, 95% CI 88.1% – 96.8% versus 74.8%, 95% CI 66.6 – 81.6%, p < 0.001) and when liver-specific images were analysed (97.8%, 95% CI 92.7% – 99.5% versus 85.1%, 95% CI 76.6% – 91.0%, p < 0.001). 99mTc was also associated with a lower negative likelihood ratio (LR) (0.002, 95% CI 0.009 – 0.1 versus 0.19, 95% CI 0.12 – 0.42, p = 0.009) when evaluating hepatic lesions. Adverse events happened in 3 pts after 111In and in 2 pts after 99mTc, all grade 1. The 99mTc demonstrated a higher sensitivity overall and a better negative LR in liver-specific images compared to 111In in pts with NETs. Our findings suggest that 99mTc is an alternative to 111In and is especially useful in ruling out liver metastases. NCT02691078.
Keywords: neuroendocrine tumours, technetium TC99m, indium In111, radionuclide imaging
Correspondence to: Gustavo Werutsky
Email: gustavo.werutsky@lacog.org.br
Published: 26/07/2023
Received: 30/09/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Neuroendocrine tumours (NETs) are rare neoplasms that arise from epithelial cells with neuroendocrine features located primarily in the lungs, pancreas and gastrointestinal tissues [1, 2]. Clinical presentation and prognosis are extremely variable, from slow-growing well-differentiated disease to highly aggressive undifferentiated tumours [3]. The worldwide incidence is estimated to be around 50 cases per million inhabitants and has increased in the past decades, mostly due to better imaging [4–6].
Until the second decade of the 2000s, the reference product for scintigraphy examinations was 111In-DTPA-octreotide, as it was the only product registered worldwide, including Brazil. However, with the introduction of positron emission tomography (PET) scan with 68Ga-DOTA-octapeptides (DOTATATE, DOTATOC and DOTANOC), which provide a superior image pattern compared to those obtained by single photon emission computed tomography (SPECT), a new gold standard was established [7–12].
However, these methods have some drawbacks that affect their availability. In Brazil, 111In-DTPA-octreotide is supplied by a single institution (IPEN – Instituto de Pesquisas Energéticas e Nucleares), which is discontinuing its commercial production. Besides, it has high production costs, needs special cameras and collimators, and has long-term radioactivity that may harm patients and technicians [13]. While 68Ga-DOTA-octapeptides are still under regulatory approval by the Brazilian Health Agency (ANVISA – Agência Nacional de Vigilância Sanitária), the 68Ga labelling process requires a more costly and time-consuming process than the one with 99mTc. Furthermore, in most low- and middle-income countries (LMICs), facilities and specialised professionals able to produce and distribute radiopharmaceuticals are lacking. Finally, SPECT cameras availability is still considerably higher than PET scans worldwide. Therefore, identifying other cheaper and quickly produced radiopharmaceuticals is currently a global unmet need.
In previous studies, 99mTC-EDDA/HYNIC-TOC (99mTc marked octreotide) demonstrated similar efficacy to 111In-DTPA-octreotide (OCTREOSCAN®) in images from tumours that express somatostatin receptors (SSTRs), such as several types of NETs [14–18]. 99mTcEDDA/HYNIC-TOC has a higher affinity for SSTR2 and lower affinity for SSTR3 and SSTR5 [19]. Furthermore, 99mTc is also easily available in nuclear medicine facilities worldwide without special technologies. Thus, we conducted the Latin American Cooperative Oncology Group (LACOG) 0214 study, which aimed to evaluate the performance of scintigraphy using 99mTc-EDDA/HYNIC-TOC compared with 111In-DTPA-octreotide for the diagnosis and staging of patients with NETs.
Materials and methods
Study design and eligibility criteria
We planned a prospective diagnostic accuracy study in patients with biopsy-proven diagnoses of NETs from any location. The inclusion criteria were histological diagnosis of NETs in any stage, provided the patient had at least one visible lesion on computed tomography or magnetic resonance imaging. Patients should also have an indication of a 111In scan and be at least 18 years old. Patients should not have received somatostatin analogues in the month preceding the scan. An independent review board approved this research, and informed consent was obtained from all participants included in the study.
Study procedures
Clinical, demographic and pathological data were collected at baseline. All patients underwent vital signs assessment and basic biochemistry labs before the procedures. Patients then initially underwent the scintigraphy using 99mTc, followed by 111In scan in 2 days. Both scans were performed at the nuclear medicine facilities of the Brain Institute (BraIns). Two independent nuclear medicine physicians who were aware of the study evaluated all images.
The type of gamma camera used for the imaging process was the Forte Gamma Camera, manufactured by Philips. The matrix size was 64 × 6. SPECT/CT fusion imaging was conducted, allowing for enhanced anatomical localisation. The acquired images were analysed using the PEGASUS software. The Supplementary Information describes the protocol information.
Patients could undergo more than one pair of scans in case of re-evaluation after therapy initiation, as clinically indicated. Information was also collected regarding the number, location and intensity of lesions’ uptake.
The 99mTc-EDDA/HYNIC-TOC images were obtained using a gamma camera with low energy collimators, focused on the photopeak of the 99mTc (140.5 keV) with the symmetrical opening of 20% and injected activity of 10 mCi. We performed images of whole-body scans with SPECT 1 and 4 hours after radiopharmaceutical injection, in dorsal decubitus, with a 13 cm/minute velocity. The 111In-DTPA-octreotide images were performed in a gamma camera composed of collimators of medium energy, centred in the two photo peaks of the 111In (173 and 247 keV) with the symmetrical opening of 20% and activity injected of 6 mCi. Whole-body scan images were performed 4 and 24 hours after radiopharmaceutical injection, dorsal decubitus position, and 10 cm/minute velocity. SPECT was performed 24 hours after the injection of the radiopharmaceutical. Optional abdomen images were made after 48 hours of injection if bowel movements had interfered with image quality.
Study endpoints
The primary outcome was the diagnostic accuracy of the 99mTc-EDDA/HYNIC-TOC compared to 111In-DTPA-octreotide for diagnosis or staging of patients with NETs, irrespective of the primary site. Secondary outcomes included the number of NETs lesions visualised with radiopharmaceutical agents and safety.
Reports of acquisition-related medical complications evaluated adverse events and toxicity. Grading of adverse events was performed using the common terminology criteria for adverse events grading system.
Statistical analysis
We estimated that 39 tests would have to be performed to demonstrate sensitivity and specificity of at least 90% with an acceptable error of 10% points for 99mTC compared to 111In, with a significance level of 95% and a 10% loss and refusal rate. Categorical variables were described using count and percentage, and numerical variables were summarised using mean and SD. Binary diagnostic tests’ properties were estimated and are presented with 95% Cis, including sensitivity, specificity, positive LR and negative LR. Positive and negative predictive values were intentionally not calculated once the sample was composed of patients with the disease on conventional imaging. Thus, prevalence and predictive values are not estimable from our sample. For all comparisons of sensitivities, specificities and LRs, global comparisons using the Wald test were performed initially and followed by individual comparisons with multiple comparisons correction using the Holm [20] method. Differences in sensitivities and specificities, LRs, and their 95% Cis were calculated using the Roldán-Nofuentes and Sidaty-Regad methods [21, 22]. Analysis was performed in R, version 4.0.5, using the Compbdt package [23]. This study was registered at clinical trials with the identifier NCT02691078 and is being reported according to the last version of the Standards for Reporting Diagnostic Accuracy Studies (STARD) guidelines [24–26] from the Equator Network.
Results
Between May 2016 and July 2017, 14 scans were performed on 9 patients. Five patients performed both tests once, three patients twice, and one patient thrice. Unfortunately, the trial was stopped early due to under-enrolment. Patients were diagnosed from October 2007 to September 2016 and were examining disease re-evaluation after treatment. All nine patients were white, and five patients (55.6%) were female. The location of each patient’s primary tumour, baseline characteristics and comorbidities are described in Table 1.
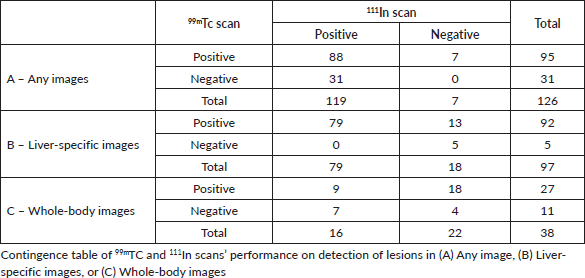
Overall, 126 lesions were identified on the 14 scans. Two scans demonstrated an uncountable number of lesions, estimated at least 20. All 14 scans were positive using 99mTc, while 4 out of the 14 scans were false negatives using 111In contingency tables presenting 99mTC and 111In scans’ performance according to image acquisition are presented in Table 2.
Estimated sensitivities, specificities and LRs are presented in Table 3. Overall, 99mTc demonstrated higher sensitivity for identifying lesions in all images (p < 0.001, 95% CI for the difference 9.54% – 27.66%) and in liver-specific images (p < 0.001, 95% for the difference: 4.72% – 20.27%). However, the difference in sensitivities of 99mTc and 111In when only whole-body images were considered was not statistically significant (p = 0.06). In addition, no statistically significant differences in specificity were found.
Table 1. Study subjects' characteristics.
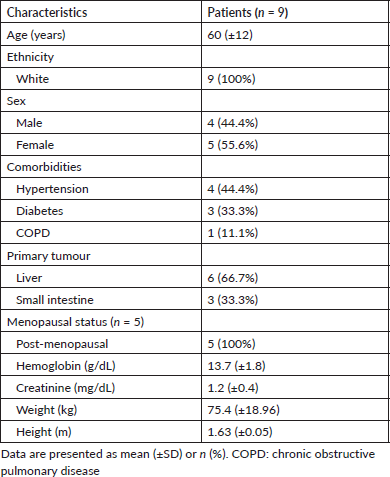
Table 2. 99mTC and 111In scans’ performance according to image acquisition.
Regarding comparing LRs, the global Wald test demonstrated a statistically significant difference in LRs of 99mTc and 111In when calculated for all images (p = 0.003) and liver-specific images (p = 0.025). However, after multiple comparison adjustments, only the difference of negative LR in liver-specific images was considered statistically significant (99mTc: 0.002, 95% CI 0.009 – 0.1 versus 111In: 0.19, 95% CI 0.12 – 0.42, p = 0.009).
Adverse events were detected in three patients after 111In scans: pruritus [1], constipation [1], vomiting and diarrhoea [1], all grade 1. Two patients experienced adverse events after 99mTc scans: pruritus [1] and vomiting and diarrhoea [1], all grade 1. No severe adverse events happened during the study.
Table 3. Performance parameters of 99mTc-EDDA/HYNIC-TOC and 111In-DTPA-OCTREOTIDE.
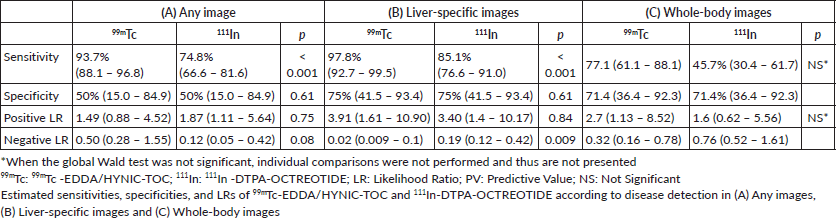
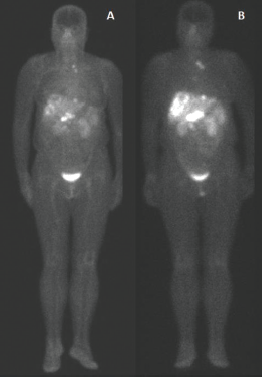
Figure 1. 99mTc-EDDA/HYNIC-TOC compared to 111In-DTPA-octreotide. Example of scintigraphy examination using (a): 111In-DTPA-octreotide and (b): 99mTc-EDDA/HYNIC-TOC in the same patient, showing a more significant number of foci of uptake using 99mTc.
In Figure 1, we have an example of the same patient evaluated by both imaging methods, showing a more significant number of foci of uptake with 99mTc-EDDA/HYNIC-TOC compared to 111In-DTPA-octreotide.
Discussion
The LACOG 0214 study was the first developed in Latin America on clinical validation of 99mTc-EDDA/HYNIC-TOC scan for diagnosing and staging patients with NET. Our results suggest that 99mTc, a cheaper and more available radiopharmaceutical, has an overall higher sensitivity than 111In, with better negative LR for liver metastasis in patients with NET.
Unfortunately, a lower-than-expected accrual resulted in early study termination and impacted our sample size. Causes for the under-enrolment cannot be ascertained. Consequently, our CI estimates for the differences in important parameters are comprehensive. For example, the 95% CI of the difference in sensitivity favouring 99mTC in all images is 9.54% – 27.66%, and in liver-specific images is 4.72% – 20.27%. However, we believe these imprecisions on the uncertainty are unfortunately inherent to the study of rare diseases such as NETs, which naturally imply smaller sample sizes and broader Cis. In this specific case, this limitation was addressed as conservatively as possible by calculating the 95% Cis with the Wald interval using the Bonett–Laplace adjustment, which was demonstrated to be superior to other methods in the previous studies [27].
Despite those limitations, the demonstration of a higher sensitivity overall, and especially a very low negative LR, of which the superior limit of the 95% CI is 0.1, supports that 99mTc may be an adequate alternative to 111In. Furthermore, it provides a better capacity to rule out somatostatin analogue uptake in the presence of liver images consistent with metastases in patients with NETs. This is even more important considering that 99mTc is associated with other beneficial characteristics, especially its availability.
Among the studies evaluating the 99mTc, a study including 173 patients with NETs who underwent 99mTc-EDDA/HYNIC-TOC scans as part of their clinical management found for pancreatic NETs a sensitivity of 94.6%, a specificity of 73.3%, and an accuracy of 90.1%. Gastrointestinal NETs found a sensitivity of 86.7%, specificity of 71.4%, and accuracy of 80.3% [28]. A study comparing 99mTc-EDDA/HYNIC-TOC with 111In-DTPA-octreotide for diagnosis of SSTR-expressing tumours in 41 patients revealed a higher sensitivity of 99mTc as compared with 111In as an imaging agent for the localisation of SSTR-expressing tumours [15].
Another study investigated 495 NETs patients and found an overall sensitivity of the 99mTc-EDDA/HYNIC-TOC of 80%, specificity of 92%, positive predictive value of 98%, negative predictive value of 47%, and accuracy of 82% [29].
A systematic review analysing studies coupled two by two comparing the peptide-based radiopharmaceuticals for PET and SPECT, 111In-DTPA-Octreotide, 99mTc-EDDA/HYNIC-TOC, 68Ga-DOTATATE/TOC and 64Cu-DOTATATE in the evaluation of NETs described true positivity rates of, respectively, 63.7%, 58.5%, 78.4% and 82.4% [30].
A literature review of 99mTc highlighted its wider availability, low cost and long decay compared to peptides labelled with 68 Ga. It concluded that the 99mTc could be proposed for a dosimetry evaluation of patients undergoing peptide receptor radionuclide therapy and for non-oncologic indications of radiolabelled somatostatin analogue (SSA) [8].
Our data reinforce that the diagnostic utility of the 99mTc-EDDA/HYNIC-TOC gain is very likely limited compared to newer but less available technologies such as 68Ga-DOTATATE PET/CT. Still, clinical validation and diagnostic accuracy of 99mTc and other markers are essential to LMICs, where the production and acquisition of radiopharmaceuticals are challenging [31, 32]. Thus, the possibility of using a more affordable and available radiopharmaceutical directly impacts care in these regions. Furthermore, even the demonstration of equivalent or non-inferior alternatives, considering other countries’ different legal constraints and regulations, impacts nuclear medicine services in LMICs due to their fragile supply chains [33]. For example, in Brazil, except for products with a half-life of fewer than 2 hours, production is a governmental monopoly, and the necessary resources are usually produced in small nuclear reactors that cannot have commercial radiopharmaceuticals. Thus, most Brazilian nuclear medicine services use foreign-produced radiopharmaceuticals and are dependent on external production, which leads to insecurity in the supply of radionuclides, as happened during the 2009 99Mo ‘crisis’ [34, 35].
Nevertheless, despite PET-CT being an increasingly used imaging method, it is also unavailable for many patients outside high-income countries [36]. For those countries which cannot afford such health expenditure, 99mTc use may be the most cost-effective strategy [33]. In the context of a rare disease, this gap may be even more significant since rare diseases such as NETs rarely have specifically designed guidance on healthcare systems and payers. Additionally, rare cancers are subject to natural limitations on clinical trial design and implementation, and this should be acknowledged when evaluating data for clinical use. Thus, a greater degree of uncertainty about therapies or diagnostic tests should also be accommodated for decision-making, as stated by the rare cancers Europe consensus panel [23].
Conclusion
Despite its early termination, the LACOG 0214 trial demonstrated a superior sensitivity of 99mTc-EDDA HYNIC-TOC compared to 111In-DTPA-octreotide in patients with NETs with a visible lesion on conventional imaging. The 99mTc was also superior regarding its negative LR when evaluating hepatic lesions, suggesting that 99mTc may be especially useful in ruling out liver metastases. Considering radiopharmaceutical acquisition is especially challenging for LMIC, the clinical validation of cheaper and more available molecules is a potential method to overcome this barrier in care.
Acknowledgments
This project would not have been possible without the financial support from the Financier of Studies and Projects (FINEP), linked to the Ministry of Science, Technology and Innovation of Brazil, within the National Plan for Science and Technology framework. The RPH Group also supported the project by providing the HYNIC-Octreotide used in this trial.
We would like to acknowledge SAS Institute Inc. for supporting our study by providing access to SAS® statistical products.
Conflicts of interest statement
Dr Moriguchi-Jeckel and Dr Werutsky report grants from Financiadora de Estudos e Projetos (FINEP) for the conduct of the study. Drs R. Madke, Viana, Nabinger, and Fernandes report employment relationships with the RPH Group. Dr. Werutsky reports personal fees from AstraZeneca, Bayer, Beigene, Daiichi Sankyo, Genentech/Roche, GSK, Lilly, MSD, Novartis, Pfizer, Sanofi, and Seattle Genetics outside the submitted work.
The other authors declare that they have no conflict of interest.
Funding statement
This project obtained financial support from the Financier of Studies and Projects (FINEP), linked to the Ministry of Science, Technology and Innovation of Brazil, within the National Plan for Science and Technology framework. This project was also supported by the RPH Group, which provided the HYNIC-Octreotide used in this trial, and by the Latin American Cooperative Oncology Group (LACOG).
Ethical statement
All procedures performed in studies involving human participants followed the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author contribution
All authors contributed substantially to the project, as defined by the Committee of Medical Journal Editors, participating in the design of the study, the data interpretation, the article's writing, the critical review of the intellectual content, and the final approval of the version to be published.
References
1. Oronsky B, Ma PC, and Morgensztern D, et al (2017) Nothing but NET: a review of neuroendocrine tumors and carcinomas Neoplasia 19(12) 991–1002 https://doi.org/10.1016/j.neo.2017.09.002 PMID: 29091800 PMCID: 5678742
2. Kunz PL (2015) Carcinoid and neuroendocrine tumors: building on success J Clin Oncol 33(16) 1855–1863 https://doi.org/10.1200/JCO.2014.60.2532
3. Rindi G, Klimstra DS, and Abedi-Ardekani B, et al (2018) A common classification framework for neuroendocrine neoplasms: an International agency for research on cancer (IARC) and World Health Organization (WHO) expert consensus proposal Mod Pathol 31(12) 1770–1786 https://doi.org/10.1038/s41379-018-0110-y PMID: 30140036 PMCID: 6265262
4. Dasari A, Shen C, and Halperin D, et al (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States JAMA Oncol 3(10) 1335–1342 https://doi.org/10.1001/jamaoncol.2017.0589
5. Hallet J, How Lim Law C, and Cukier M, et al (2015) Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes Cancer 121 589–597 https://doi.org/10.1002/cncr.29099
6. Mocellin S and Nitti D (2013) Gastrointestinal carcinoid: epidemiological and survival evidence from a large population-based study (n= 25 531) Ann Oncol 24(12) 3040–3044 https://doi.org/10.1093/annonc/mdt377 PMID: 24050954
7. Kwekkeboom DJ, Kam BL, and Van Essen M, et al (2010) Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors Endocr Relat Cancer 17 R53–R73 https://doi.org/10.1677/ERC-09-0078
8. Sadowski SM, Neychev V, and Millo C, et al (2016) Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites J Clin Oncol 34(6) 588 https://doi.org/10.1200/JCO.2015.64.0987 PMCID: 4872030
9. Öberg K (2012) Gallium-68 somatostatin receptor PET/CT: is it time to replace 111indium DTPA octreotide for patients with neuroendocrine tumors? Endocrine 42(1) 3–4 https://doi.org/10.1007/s12020-012-9681-4 PMID: 22562721
10. Wong KK, Cahill JM, and Frey KA, et al (2010) Incremental value of 111-in pentetreotide SPECT/CT fusion imaging of neuroendocrine tumors Acad Radiol 17(3) 291–297 https://doi.org/10.1016/j.acra.2009.08.015
11. Sainz-Esteban A, Olmos R, and González-Sagrado M, et al (2015) Contribution of 111In-pentetreotide SPECT/CT imaging to conventional somatostatin receptor scintigraphy in the detection of neuroendocrine tumours Nucl Med Commun 36(3) 251–259 https://doi.org/10.1097/MNM.0000000000000239
12. Krausz Y, Keidar Z, and Kogan I, et al (2003) SPECT/CT hybrid imaging with 111In‐pentetreotide in assessment of neuroendocrine tumours Clin Endocrinol 59(5) 565–573 https://doi.org/10.1046/j.1365-2265.2003.01885.x
13. Shah MH, Goldner WS, and Halfdanarson TR, et al (2018) NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018 J Nat Compr Cancer Netw 16(6) 693–702 https://doi.org/10.6004/jnccn.2018.0056
14. Artiko V, Sobic-Saranovic D, and Pavlovic S, et al (2012) The clinical value of scintigraphy of neuroendocrine tumors using (99m) Tc-HYNIC-TOC J BUON 17(3) 537–542 PMID: 23033296
15. Gabriel M, Decristoforo C, and Donnemiller E, et al (2003) An intrapatient comparison of 99mTc-EDDA/HYNIC-TOC with 111In-DTPA-octreotide for diagnosis of somatostatin receptor-expressing tumors J Nucl Med 44(5) 708–716
16. Garai I, Barna S, and Nagy G, et al (2016) Limitations and pitfalls of 99mTc-EDDA/HYNIC-TOC (Tektrotyd) scintigraphy Nucl Med Rev 19(2) 93–98 https://doi.org/10.5603/NMR.2016.0019
17. Nilsson O, Ko ̈lby L, and Wa ̈ngberg B, et al (1998) Comparative studies on the expression of somatostatin receptor subtypes, outcome of octreotide scintigraphy and response to octreotide treatment in patients with carcinoid tumours Br J Cancer 77 632–637 https://doi.org/10.1038/bjc.1998.101 PMID: 9484822 PMCID: 2149934
18. Reubi JC, Hacki WH, and Lamberts SW (1987) Hormone-producing gastrointestinal tumors contain a high density of somatostatin receptors J Clin Endocrinol Metab 65 1127–1134 https://doi.org/10.1210/jcem-65-6-1127 PMID: 2824549
19. Sergieva S, Robev B, and Dimcheva M, et al (2016) Clinical application of SPECT-CT with 99mTc-tektrotyd in bronchial and thymic neuroendocrine tumors (NETs) Nucl Med Rev 19 81–87 https://doi.org/10.5603/NMR.2016.0017
20. Holm S (1979) A simple sequentially rejective multiple test procedure Scand J Stat 6 65–70
21. Roldán-Nofuentes JA and Sidaty-Regad SB (2019) Recommended methods to compare the accuracy of two binary diagnostic tests subject to a paired design J Stat Comput Simul 89(14) 2621–2644 https://doi.org/10.1080/00949655.2019.1628234
22. Nofuentes JA and Del Castillo JD (2007) Comparison of the likelihood ratios of two binary diagnostic tests in paired designs Stat Med 26(22) 4179–4201 https://doi.org/10.1002/sim.2850 PMID: 17357992
23. Casali PG, Bruzzi P, and Bogaerts J, et al (2015) Rare cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: a European consensus position paper Ann Oncol 26(2) 300–306 https://doi.org/10.1093/annonc/mdu459 PMCID: 4304377
24. Bossuyt PM, Reitsma JB, and Bruns DE, et al (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative Ann Intern Med 138(1) 40–44 https://doi.org/10.7326/0003-4819-138-1-200301070-00010 PMID: 12513043
25. Bossuyt PM, Reitsma JB, and Bruns DE, et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies [Online] BMJ 351 h5527 https://doi.org/10.1136/bmj.h5527
26. Cohen JF, Korevaar DA, and Gatsonis CA, et al (2017) STARD for abstracts: essential items for reporting diagnostic accuracy studies in journal or conference abstracts bmj 358 j3751 https://doi.org/10.1136/bmj.j3751
27. Fagerl and MW, Lydersen S, and Laake P (2014) Recommended tests and confidence intervals for paired binomial proportions Stat Med 33(16) 2850–2875 https://doi.org/10.1002/sim.6148 PMID: 24648355
28. Gherghe M, Lazăr AM, and Stanciu AE, et al (2022) The new radiolabeled peptide 99mTcEDDA/HYNIC-TOC: is it a feasible choice for diagnosing gastroenteropancreatic NETs? Cancers (Basel) 14(11) 2725 https://doi.org/10.3390/cancers14112725 PMID: 35681704 PMCID: 9179571
29. Artiko V, Afgan A, and Petrović J, et al (2016) Evaluation of neuroendocrine tumors with 99mTc-EDDA/HYNIC TOC Nucl Med Rev Cent East Eur 19(2) 99–103 https://doi.org/10.5603/NMR.2016.0020 PMID: 27479786
30. Poletto G, Cecchin D, and Sperti S, et al (2022) Head-to-Head comparison between peptide-based radiopharmaceutical for PET and SPECT in the evaluation of neuroendocrine tumors: a systematic review Curr Issues Mol Biol 44(11) 5516–5530 https://doi.org/10.3390/cimb44110373 PMID: 36354685 PMCID: 9689511
31. Santos-Oliveira R, Weiss Smith S, and Souza Albernaz MD, et al (2011) Surveillance of radiopharmaceuticals in Latin American: an alert Rev Esp Med Nucl (Ed. impr.) 30(2) 134–136 https://doi.org/10.1016/j.remn.2010.10.010
32. Rezende dos Reis SR, Oliveira T, and Cavancanti da Silva LF, et al (2021) PET radiopharmaceuticals and PET/CT technology: comparative numbers of Brazil, India, Canada and Latin America www.austinpublishinggroup.com Date accessed: 08/05/21
33. Briganti V, Cuccurullo V, and Berti V, et al (2020) 99mTc-EDDA/HYNIC-TOC is a new opportunity in neuroendocrine tumors of the lung (and in other malignant and benign pulmonary diseases) Curr Radiopharm 13(3) 166–176 https://doi.org/10.2174/1874471013666191230143610
34. Osso Jr JA, Dias CRBR, and Brambilla TP, et al (2013) Production of 99Mo at IPEN-CNEN/SP-Brazil https://www.ipen.br/biblioteca/2013/eventos/19834.pdf
35. Gould P (2009) Medical isotope shortage reaches crisis level; robust solutions sought urgently to shore up fragile supply chain Nature 460(7253) 312–314 https://doi.org/10.1038/460312a
36. Verduzco-Aguirre HC, Lopes G, and Soto-Perez-De-Celis E (2019) Implementation of diagnostic resources for cancer in developing countries: a focus on PET/CT Ecancermedicalscience 13 https://doi.org/10.3332/ecancer.2019.ed87 PMID: 30915165 PMCID: 6390832
Supplementary information
Study protocol
Preparation of the patient for the exam for the two radiopharmaceuticals:
The patient must fast for 4 hours for solids before administration of the radiopharmaceutical, and must remain well hydrated before and after the examination, in order to reduce the radiation dose. The use of non-radiolabeled somatostatin analogues may reduce the sensitivity of the method. Therefore, the exams will be carried out just before the administration of long-acting formulas. Short-acting formulas will be suspended 24 hours before the exam.
Instrumentation
Radiopharmaceutical: HYNICTOC-EDDA-99mTc
The images will be performed in the same gamma-camera; with low-energy collimators, centred on the Tc-99m photopeak (140.5 keV) with a symmetrical aperture of 20%. The injected activity will be 10 mCi.
Image acquisition protocol:
• Scanning images of the entire body will be performed 1 and 4 hours after the injection of the radiopharmaceutical, in dorsal decubitus, with arms down, with a 1,024 × 512 matrix, at a speed of 13 cm/minute.
• Tomographic images (SPECT) of the areas of interest will be taken 4 hours after the injection of the radiopharmaceutical in dorsal decubitus, with a 64 × 64 matrix, 64 projections of 30 seconds and zoom of 1.
Radiopharmaceutical: OCTREOTIDEO – DTPA – In-111
The images will be taken in a gamma-camera composed of medium-energy collimators, centred on the two In-111 photopeaks (173 and 247 keV) with a symmetrical aperture of 20%. The injected activity will be 6 mCi.
Image acquisition protocol:
• Scanning images of the entire body will be performed 4 and 24 hours after the injection of the radiopharmaceutical, in dorsal decubitus, with arms down, matrix 1,024 × 512 and speed of 10 cm/minute.
• Tomographic images (SPECT) of the areas of interest will be taken 24 hours after the injection of the radiopharmaceutical in the supine position, with a 64 × 64 matrix, 64 projections of 35 seconds and a zoom of 1.
• Planar images of the abdomen, of 48 hours, are optional, in case there is interference of intestinal activity making it difficult to interpret the 24 hours images. In this case, they will be performed with 500,000 counts and a 128 × 128 matrix.