Unresectable metastatic colorectal cancer in fit patients – a practical algorithm of treatment sequencing from the Brazilian Group of Gastrointestinal Tumours (GTG)
Renata D’Alpino Peixoto1, Anelisa K Coutinho2, Gabriel Prolla3, Rui F Weschenfelder4 and Rachel Riechelmann5
1Centro Paulista de Oncologia (Oncoclínicas), São Paulo 04538-132, Brazil
2Clínica AMO (DASA), Salvador 41950-640, Brazil
3Oncoclínicas, Porto Alegre 90570-020, Brazil
4Hospital Moinhos de Vento, Porto Alegre 90035-000, Brazil
5AC Camargo Cancer Center, São Paulo 01509-001, Brazil
Abstract
Recent advances in biomarker-driven therapies have changed the landscape of unresectable metastatic colorectal cancer (mCRC) and brought not only access issues but also difficulties for the treating physician (especially generalist oncologists) in choosing the most suitable treatment for each individual patient. This manuscript proposes an algorithm developed by The Brazilian Group of Gastrointestinal Tumours with the aim of bringing easy-to-follow steps in the management of unresectable mCRC. The algorithm is based on evidence for fit patients to facilitate therapeutic decisions in the clinical practice and assumes that there are no access and resource limitations.
Keywords: algorithm, metastatic colorectal cancer, colon cancer treatment
Correspondence to: Renata D’Alpino Peixoto
Email: renatadalpino@gmail.com
Published: 02/05/2023
Received: 06/02/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
According to GLOBOCAN 2020, approximately 10 million cancer deaths occur worldwide per year and colorectal cancer (CRC) accounts for 9.4% of them, representing the second leading cause of cancer mortality [1]. Thereby, metastatic CRC (mCRC) poses an immense public health challenge and deserves further attention. Recent advances in biomarker-driven therapies changed the landscape of unresectable mCRC and brought not only access issues but also difficulties for the treating physician (especially generalist oncologists) in choosing the most suitable treatment for each individual patient [2].
Unfortunately, most guidelines are not straightforward when it comes to deciding the treatment sequencing of mCRC in algorithms. Most of them focus mainly on the first-line settings while others have yet not incorporated recent advances [3–5]. The Brazilian Group of Gastrointestinal Tumours (GTG) recognises the difficulty of bringing easy-to-follow steps in the management of unresectable mCRC and proposes an ideal algorithm based on evidence for fit patients to facilitate therapeutic decisions in the clinical practice, although limitations in depicting all possible clinical scenarios need to be recognised, such as maintenance and locoregional therapies (Figure 1). Our algorithm was built on the assumption that there are no access and resource limitations. Therefore, it should not be considered a regulatory guideline. We recognise that in most parts of the world many of the options recommended are not available. In this case, one should move to the next step in this algorithm. In addition, whenever possible, patients should be encouraged to participate in clinical trials.
Approximately 3%–5% of mCRC have microsatellite instability (MSI-high)/deficient mismatch repair and are currently best treated with first-line immunotherapy. The highest level of evidence we have so far is with pembrolizumab in monotherapy [6]. However, the combination of ipilimumab and nivolumab is also promising and approved in many countries [7]. After progression to first-line immunotherapy in MSI-H, we prefer to offer a second-line doublet (fluoropyrimidine with irinotecan or oxaliplatin) with bevacizumab if raf murine sarcoma viral oncogene homolog B1 (BRAF) wild-type, especially if rat sarcoma (RAS) mutated or right-sided tumours. Recent data suggest certain resistance to anti-EGFR agents in MSI-H tumours [8]. However, it is also possible to offer a doublet with anti-EGFR in the second-line setting if RAS wild-type and left-sided since data of resistance to anti-EGFR in MSI-H tumours is still scarce. Since MSI-H mCRC may coexist with BRAF V600E mutations, a BRAF inhibitor combined with cetuximab may be offered as second-line regimen when this is the case [9]. Further lines of therapy for MSI-H tumours follow the same rules as for microsatellite stable (MSS) mCRC. When the patient progresses on second-line doublet therapy with bevacizumab and maintains good performance status, a further decision is based on molecular profiling. After that, trifluridine-tipiracil (TFD/TPI) with bevacizumab (preferable) or regorafenib are options [10, 11].
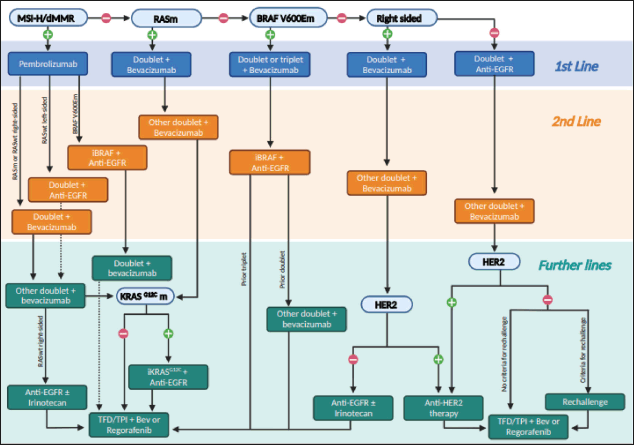
Figure 1. An algorithm for treatment decisions in unresectable mCRC for each line of therapy.
For patients with MSS tumours harbouring a RAS mutation, our recommended first-line option is a doublet (FOLFOX, CAPOX or FOLFIRI) with bevacizumab, although eventually a triplet (FOLFOXIRI) with bevacizumab may be used in patients with a high volume of disease and need of response [12]. It is reasonable to discontinue oxaliplatin or irinotecan after a period of induction therapy (approximately 3–4 months) and continue maintenance single-agent fluoropyrimidine with bevacizumab until the progression of the disease occurs. At that time, reintroduction of the first-line therapy or moving to a second-line therapy can be discussed. After progression on a first-line doublet with bevacizumab, we would change to the alternative second-line doublet while maintaining bevacizumab if it proved to be beneficial in first line [13] (if combined with second-line FOLFIRI, ramucirumab or aflibercept may replace bevacizumab) [14, 15]. If RAS mutation is in K-RAS G12C (approximately 4% of the cases), third-line therapy with a KRAS G12C inhibitor plus an anti-EGFR may be used [16, 17], followed by TFD/TPI and bevacizumab (preferable) or regorafenib on progression [10, 11]. In the case of other RAS mutations, third-line treatment typically involves either TFD/TPI with bevacizumab or regorafenib [10, 11].
For patients with MSS BRAF V600E mutations, although debatable, we tend to offer more aggressive first-line options, such as a triplet with bevacizumab when tolerable [12]. However, a first-line doublet with bevacizumab is also reasonable. Anti-BRAF agents in combination with anti-EGFR are currently offered in the second or later-lines setting, although they are currently being studied as first-line options. It is important to mention that the addition of binimetinib to cetuximab and vemurafenib also yielded benefit when compared to ireinotecan-based chemotherapy and cetuximab in the phase III BEACON trial. However, we prefer to offer cetuximab and encorafenib since this combination performed similarly to encorafenib, cetuximab and binimetinib [9]. For those who progressed both on first-line triplet with bevacizumab and on second-line anti-EGFR plus anti-BRAF, third-line options include either TFD/TPI with bevacizumab (preferable) or regorafenib [10, 11]. For those who used a doublet in the first line setting, the alternative doublet may also be used before the oral drugs.
When the patient has an MSS, RAS and BRAF wild-type tumour, the next important question to answer is the location of the primary lesion. For right-sided tumours, a doublet with bevacizumab is typically the first-line option followed by the alternative doublet with bevacizumab (or other anti-angiogenic) on progression [18, 19]. In these cases, an anti-EGFR with or without irinotecan is used in the third-line setting, followed by TFD/TPI with bevacizumab or regorafenib in later lines for human epidermal growth factor receptor 2 (HER2)-negative tumours and by anti-HER strategies for HER2-positive tumours. Many anti-HER2 agents in combinations have been studied in mCRC, but so far, there is no gold standard [20, 21]. There is even evidence for trastuzumab-deruxtecan or tucatinib with trastuzumab after failure to other anti-HER2 agents and that latter would be our choice of treatment for metastatic HER + CRC, when available [22, 23].
On the other hand, left-sided tumours should be treated with a doublet with an anti-EGFR in the first-line setting [18, 19]. On progression, the alternative doublet with bevacizumab should be used. The third-line option depends on HER2 status and on clinical and/or liquid biopsy-driven results in order to decide whether rechallenge with chemotherapy with anti-EGFR therapy could be useful. If HER2 positive, we suggest anti-HER2 strategies [20–22]. If negative, we would look into progression-free survival on first-line doublet with anti-EGFR. Those patients who benefited from anti-EGFR therapy and, on progression, stayed at least 4 months away from anti-EGFR in the second-line setting, could receive rechallenge with chemotherapy plus an anti-EGFR agent, especially if liquid biopsy rules out RAS mutations (or other anti-EGFR resistance alterations) [24, 25]. However, if those criteria cannot be fulfilled and HER2 is negative, we prefer TFD/TPI with bevacizumab (preferable) or regorafenib as a third-line therapy. More recently, fruquintinib has demonstrated activity in the refractory setting, even after failure to TFD/TPI plus bevacizumab and/or regorafenib, regardless of the molecular profile, and may be an option when available [26].
Neurotrophic tyrosine receptor kinase (NTRK) fusions, although very rare among mCRC, is also a target. NTRK inhibitors, such as larotrectinib, may be used after at least one prior line of therapy for those patients who harbour NTRK fusions, which is more commonly seen in MSI-H tumours without RAS or BRAF mutations [27]. In addition, rearranged during transfection (RET) fusions may also be targeted by selpercatinib [28].
Conclusion
With this algorithm, GTG believes that most scenarios of unresectable mCRC in fit patients are covered and have the potential to help clinicians in therapeutic decisions. However, access and resource limitations must be considered in clinical practice.
Acknowledgments
The authors acknowledge Maria Cecilia Mathias-Machado for her help in the elaboration of Figure 1.
Conflicts of interest
The authors declare no conflict of interest for this manuscript.
Financial declaration
None.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Guren MG (2019) The global challenge of colorectal cancer Lancet Gastroenterol Hepatol 4(12) 894–895 https://doi.org/10.1016/S2468-1253(19)30329-2 PMID: 31648973
3. Cervantes A, Adam R, and Roselló S, et al (2023) Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up Ann Oncol 34(1) 10–32 https://doi.org/10.1016/j.annonc.2022.10.003
4. Benson AB, Venook AP, and Al-Hawary MM, et al (2021) Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology J Natl Compr Cancer Netw 19(3) 329–359 https://doi.org/10.6004/jnccn.2021.0012
5. Yoshino T, Arnold D, and Taniguchi H, et al (2018) Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS Ann Oncol 29(1) 44–70 https://doi.org/10.1093/annonc/mdx738
6. Diaz LA, Shiu KK, and Kim TW, et al (2022) Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study Lancet Oncol 23(5) 659–670 https://doi.org/10.1016/S1470-2045(22)00197-8 PMID: 35427471 PMCID: 9533375
7. Lenz HJ, Van Cutsem E, and Luisa Limon M, et al (2022) First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II checkMate 142 study J Clin Oncol 40(2) 161–170 https://doi.org/10.1200/JCO.21.01015
8. Innocenti F, Ou FS, and Qu X, et al (2019) Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome J Clin Oncol 37(14) 1217–1227 https://doi.org/10.1200/JCO.18.01798 PMID: 30865548 PMCID: 6506418
9. Kopetz S, Grothey A, and Yaeger R, et al (2019) Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer N Engl J Med 381(17) 1632–1643 https://doi.org/10.1056/NEJMoa1908075 PMID: 31566309
10. Tabernero J, Prager GW, and Fakih M, et al (2023) Trifluridine/tipiracil plus bevacizumab for third-line treatment of refractory metastatic colorectal cancer: the phase 3 randomized SUNLIGHT study J Clin Oncol 41(4_suppl) 4 https://doi.org/10.1200/JCO.2023.41.4_suppl.4
11. Grothey A, Van Cutsem E, and Sobrero A, et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial Lancet Lond Engl 381(9863) 303–312 https://doi.org/10.1016/S0140-6736(12)61900-X
12. Loupakis F, Cremolini C, and Masi G, et al (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer N Engl J Med 371(17) 1609–1618 https://doi.org/10.1056/NEJMoa1403108 PMID: 25337750
13. Bennouna J, Sastre J, and Arnold D, et al (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial Lancet Oncol 14(1) 29–37 https://doi.org/10.1016/S1470-2045(12)70477-1
14. Tabernero J, Yoshino T, and Cohn AL, et al (2015) Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study Lancet Oncol 16(5) 499–508 https://doi.org/10.1016/S1470-2045(15)70127-0 PMID: 25877855
15. Van Cutsem E, Tabernero J, and Lakomy R, et al (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen J Clin Oncol 30(28) 3499–3506 https://doi.org/10.1200/JCO.2012.42.8201 PMID: 22949147
16. Ou SHI, Jänne PA, and Leal TA, et al (2022) First-in-human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRASG12C solid tumors (KRYSTAL-1) J Clin Oncol 40(23) 2530–2538 https://doi.org/10.1200/JCO.21.02752 PMID: 35167329 PMCID: 9362872
17. Fakih MG, Kopetz S, and Kuboki Y, et al (2022) Sotorasib for previously treated colorectal cancers with KRASG12C mutation (Code-BreaK100): a prespecified analysis of a single-arm, phase 2 trial Lancet Oncol 23(1) 115–124 https://doi.org/10.1016/S1470-2045(21)00605-7
18. Holch JW, Ricard I, and Stintzing S, et al (1990) The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials Eur J Cancer Oxf Engl 70 87–98 https://doi.org/10.1016/j.ejca.2016.10.007
19. Yoshino T, Watanabe J, and Shitara K, et al (2022) Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): results from the phase 3 PARADIGM trial J Clin Oncol 40(17_suppl) LBA1 https://doi.org/10.1200/JCO.2022.40.17_suppl.LBA1
20. Sartore-Bianchi A, Trusolino L, and Martino C, et al (2016) Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial Lancet Oncol 17(6) 738–746 https://doi.org/10.1016/S1470-2045(16)00150-9 PMID: 27108243
21. Hainsworth JD, Meric-Bernstam F, and Swanton C, et al (2018) Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study J Clin Oncol 36(6) 536–542 https://doi.org/10.1200/JCO.2017.75.3780 PMID: 29320312
22. Siena S, Di Bartolomeo M, and Raghav K, et al (2021) Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial Lancet Oncol 22(6) 779–789 https://doi.org/10.1016/S1470-2045(21)00086-3 PMID: 33961795
23. Strickler J, Cercek A, and Siena S, et al (2022) LBA-2 primary analysis of MOUNTAINEER: a phase 2 study of tucatinib and trastuzumab for HER2-positive mCRC Ann Oncol 33 S375–S376 https://doi.org/10.1016/j.annonc.2022.04.440
24. Cremolini C, Rossini D, and Dell’Aquila E, et al (2019) Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial JAMA Oncol 5(3) 343–350 https://doi.org/10.1001/jamaoncol.2018.5080 PMCID: 6439839
25. Sartore-Bianchi A, Pietrantonio F, and Lonardi S, et al (2022) Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial Nat Med 28(8) 1612–1618 https://doi.org/10.1038/s41591-022-01886-0 PMID: 35915157 PMCID: 9386661
26. Dasari NA, Lonardi S, and Garcia-Carbonero R, et al (2022) LBA25 FRESCO-2: a global phase III multiregional clinical trial (MRCT) evaluating the efficacy and safety of fruquintinib in patients with refractory metastatic colorectal cancer Ann Oncol 33 S1391–S1392 https://doi.org/10.1016/j.annonc.2022.08.021
27. Drilon A, Laetsch TW, and Kummar S, et al (2018) Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children N Engl J Med 378(8) 731–739 https://doi.org/10.1056/NEJMoa1714448 PMID: 29466156 PMCID: 5857389
28. Subbiah V, Wolf J, and Konda B, et al (2022) Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial Lancet Oncol 23(10) 1261–1273 https://doi.org/10.1016/S1470-2045(22)00541-1 PMID: 36108661