Evaluation of quality of life of gastrointestinal cancer patients presenting to a tertiary care hospital in Pakistan
Muhammad Tayyab H Siddiqui1, Muhammad Rizwan Khan2, Adnan Jawaid2, Fatima Shaukat3 and Nida Zahid2
1Department of Surgery, Patel Hospital, Karachi 75300, Pakistan
2Department of Surgery, Aga Khan University Hospital, Karachi 74000, Pakistan
3Department of Radiation Oncology, Cyber-knife and Tomotherapy Centre, Jinnah Post-Graduate Medical Center, Karachi 75510, Pakistan
Abstract
Introduction: Quality of life (QOL) appraisal is a meaningful method of outcomes assessment in patients with gastrointestinal (GI) cancer. The aim of our study was to evaluate QOL of patients suffering from GI cancer, who underwent treatment at Aga Khan University Hospital (AKUH), Karachi, Pakistan.
Methods: It was a cross-sectional study. A total of 158 adults from December 2020 to May 2021 were included in the study. The EORTC QLQ-C30, validated in Urdu (Pakistan) version, was used to assess the QOL of the participants. Mean QOL scores were calculated and compared with threshold of clinical importance (TCI). Multivariate analysis was done to analyse the correlation between independent factors and QOL scores. A p value of <0.05 was considered as significant.
Results: Mean age of the study participants was 54.5 ± 13 years. Majority were male, married and living in combined family system. Most common GI cancer was colorectal (61%) followed by stomach (33.5%); and the most frequent stage at presentation was stage III (40%). Global QOL score was found to be 65.48 ± 1.78. Among functioning scales, role functioning, social functioning, emotional functioning and cognitive functioning were found to be above TCI, whereas physical functioning was found to be below TCI. Among symptom scores, fatigue, pain, dyspnoea, insomnia, appetite loss, constipation and diarrhoea were found to be below TCI, whereas nausea/vomiting and financial impact were found to be above TCI. Multivariate analysis revealed that history of surgery had a positive association (p < 0.001), while being on treatment (p = 0.001) and having a stoma (p = 0.038) had a negative impact on global QOL.
Conclusion: This is the first study to evaluate the QOL scores in GI cancer patients in Pakistan. There is a need to identify the reasons for low physical functioning score and explore means to mitigate symptoms scores above TCI in our population.
Keywords: quality of life, gastrointestinal cancer, EORTC QLQ-C30
Correspondence to: M Tayyab H Siddiqui
Email: tayyabsiddiqui87@gmail.com
Published: 03/04/2023
Received: 08/11/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cancer is a leading cause of death in many countries and expected to be the most common reason of decreasing life expectancy worldwide over the course of this century [1]. Likewise, the rate of cancer is escalating in Pakistan, with an expected rise in the incident rate of 150,000 cases each year [2]. Gastrointestinal (GI) cancers allude to the malignancies emerging in the GI tract and accessory organs involved in digestion [3].
GI cancer and its treatment have a noteworthy impact on wellbeing related to the QOL [4]. Chemotherapy instigated nausea and emesis, surgical procedure related altered bowels habits and need of dietary modifications, social and emotional trauma because of stoma bag and skin irritation post radiation are few of many sufferings faced by GI cancer patients. Calculating morbidity, mortality, recurrence rate, overall and disease free survival are the classic methods to assess the success of a treatment plan.
Over the years, quality of life (QOL) appraisal has assumed a significant importance in the assessment of chronic disease, especially the cancer clinical preliminaries [5]. As of late, different tools have been created to quantify significant result factors for evaluating the QOL in cancer patients, especially self-managed questionnaires [6]. The European Organization of Research and Treatment of Cancer (EORTC) has built up a centre QOL questionnaire QLQ-C30, which is the most generally utilised cancer questionnaire [7]. EORTC-QLQ-C30 is deciphered and approved in excess of 60 dialects including Urdu [7]. Numerous clinical studies performed to assess the QOL in patients suffering from cancer have been conducted which demonstrate that different elements like age, co-morbid conditions and education have an integral impact on QOL of patients diagnosed with cancer [8].
In Pakistan, only few studies to assess QOL have been conducted in patients with breast cancer and patients undergoing chemotherapy [9–11]. However, no study has so far been reported to examine the overall QOL in GI cancer patients and its related factors. The objectives of our study were to evaluate the QOL of patients suffering from GI cancer using EORTC QLQ-C30 (Urdu) and to identify factors associated with QOL of GI cancer patients. It is a basic prerequisite to assess QOL of GI cancer sufferers, to make fitting strides towards their ideal care.
Methodology
An analytical cross-sectional study was designed and Institutional Ethical Review Committee (ERC) approval was taken before collecting the data. Patients aged 18 years or above, who were receiving or previously received any form of treatment for GI tumour at AKUH and willing to participate in the study were approached in surgery and oncology clinics. Patients with prior history of malignancy other than GI cancer, patients with psychiatric disorders or those who are unable to understand English or Urdu were excluded. Written and verbal consent was taken from eligible patients who were willing to participate. Each participant was provided with two self-filled questionnaires. Either patient or caregiver filled the questionnaire, help was provided by one of the trained team member whenever needed. Socio-demographic and factors associated with QOL like age, gender, education, marital status, disease status and treatment received were collected in a self-designed questionnaire. For QOL assessment, EORTC QLQ-C30 Urdu (Pakistan) version was used.
The EORTC QLQ-C30, validated in Urdu (Pakistan) version is a core cancer specific questionnaire. It consist of 30 items measuring functioning scales (physical, social, role, cognitive and emotional), disease and treatment related symptoms scales (fatigue, nausea/vomiting, pain, dyspnoea, sleep disturbance, appetite loss, constipation, diarrhoea and financial impact) and global QOL. The scores marked by each participant for every scale were converted to 0–100 scales according to EORTC scoring manual [12]. For functioning scale and global QOL, higher score indicates better QOL whereas for symptom scales higher scores indicate higher symptom burden, i.e. worse QOL. All measured scores for functioning scales and symptom scales were compared with threshold of clinical importance calculated by EORTC [13].
The sample size was calculated based on previously reported estimates for QOL among patients with GI cancers. It has been calculated using one population mean formula, based on a SD range of 7.5%–25.9%, 5% level of significance with precision of 5 and by adjusting the sample size for 10% non-response rate. The minimum sample size is estimated to be 113 [14–17].
Data were analysed on STATA version 15. The quantitative variables are reported as mean± SD/ median (IQR). The qualitative variables are reported as frequency and percentages. Mean scores calculated for different variables of QOL . To analyse the correlations between the independent factors and the QOL scales, general linear model (GLM) multivariate analysis of variance (MANOVA) is performed. Univariate and multivariate Wilks is reported to test the impact of each variable included in the model. To assess the relationship of global QOL with the independent factors, linear regression modelling is performed and unadjusted and adjusted beta coefficient is reported with their 95% CI. A p value of <0.05 is considered as significant throughout the study.
Results
Overall, 158 GI cancer patients participated in this study. Table 1 represents the demographic profile of participants. Mean age of the study participants was 54.5 ± 13 years. Majority were male (73%), married (86%), living in combined family system (55%). Only 65 (41%) of the study participants were employed. Literacy status revealed that 77 (48.7%) participants were graduates, while 30 (19%) were either illiterate or just able to write their names. Urdu (29.7%) was the most commonly speaking language in study participants followed by Sindhi (25.3%).
Table 1. Demographic profile of GI cancer patients.
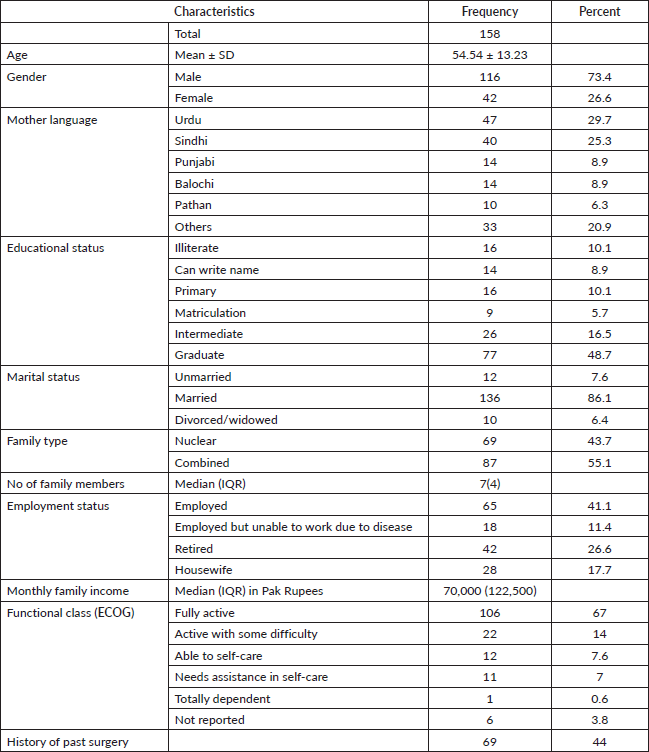
Table 2 summarises clinical characteristics and treatment received by study participants. Most common cancer included colon 76 (48%) followed by stomach 53 (33.5%). Stage III was the most frequent stage at presentation (40%). Surgical intervention was performed in 127 (80.4%) patients, out of which 39 (24.7%) had a stoma. Majority of the patients (61.4%) were on treatment at the time of participation in the study. The most common comorbid found in our participants was hypertension 60 (38%) followed by diabetes mellitus 44 (27.8%).
The overall global QOL score was 65.48 ± 1.78. Individual domain scores are presented in Table 3 with TCI scores for comparison. It was observed that among functioning scales, role functioning, social functioning, emotional functioning and cognitive functioning were found to be above TCI whereas physical functioning was found to be below TCI. Among symptom scores; fatigue, pain, dyspnoea, sleep disturbance, loss of appetite, constipation, diarrhoea were found to be below TCI, whereas nausea/vomiting and financial impact were found to be above TCI.
Linear regression model was used to assess the association between factors affecting global QOL. Table 4 shows the univariate and multivariate analysis. Multivariate analysis showed that patients who had surgery (p < 0.001) had a significant better global QOL whereas those patients who had a stoma (p = 0.001) or who are currently on treatment (p = 0.038) had a significantly worse global QOL.
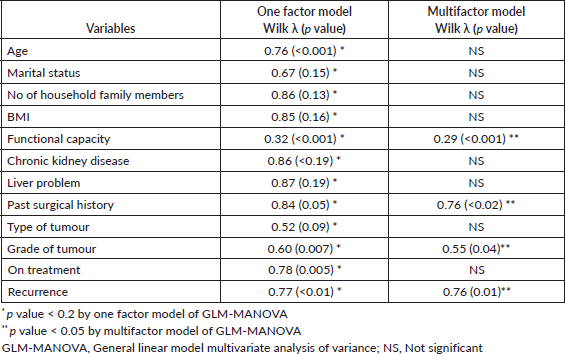
GLM-MANOVA was used to determine the association between the independent variables and the dependent variables (15 scales of QLQ–C30). On the one-factor model, 12 variables were associated with the overall outcome of QLQ–C30 with p < 0.20. These 12 variables were entered into the multifactor model analysis. Variables significantly associated on the multifactor model were functional capacity, past surgical history, grade of tumour and recurrence of tumour (all p < 0.05). Results of the GLM-MANOVA are shown in Table 5.
Table 2. Clinical characteristics and treatment received.
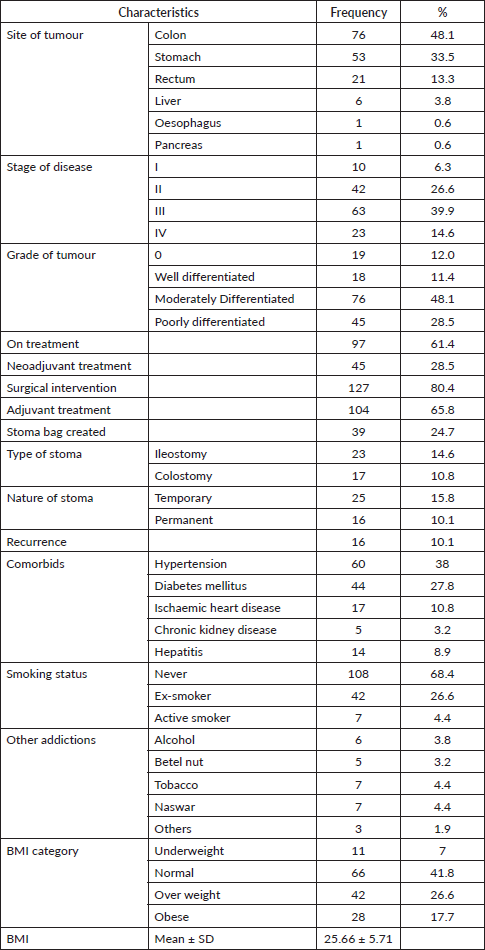
Table 3. QOL score and comparison with threshold of clinical significance (TCI).
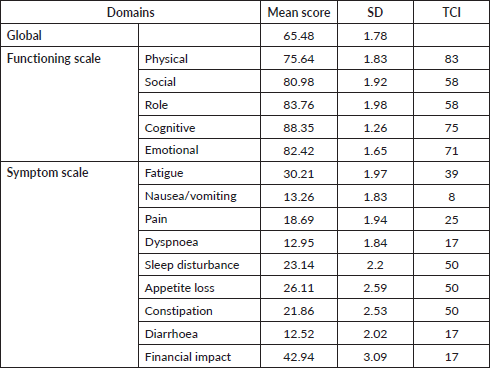
Table 4. Univariate and multivariate analysis for assessing factors associated with Global QOL.
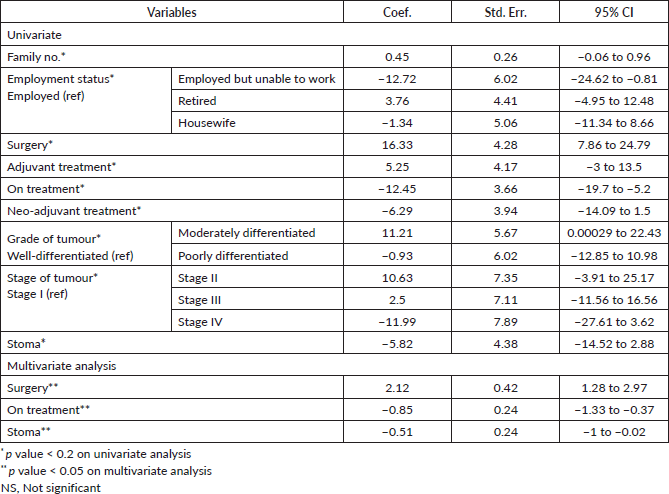
Table 5. Association of clinico-demographic variables with QLQ-C30 using GLM-MANOVA.
Discussion
We analysed the QOL of GI cancer patients using Urdu version of EORTC QLQ C30 tool, one of the most widely used patient reported outcome tool to assess health related QOL [18]. The mean global QOL score was 65.48 ± 1.78. Our Global QOL score was found to be comparable to international studies on GI cancer patients ranging from (55–76.8) [19–22]. When we compared our global QOL score with other studies on Pakistani population, we observe a better score in our GI cancer patients as compared to patients with blood cancer (25.95), breast cancer (48.33) and with different combination of cancer on chemotherapy (57.37) [9–11]. These differences can be explained by complete different spectrum of symptomatology, treatment options especially chemotherapeutic drugs and problems associated with those treatment modalities.
Among the five functioning scales, physical functioning was the most commonly affected area in our patients with a mean score of 75.63 ± 1.83 which is comparable to the scores from the international data on GI cancers which ranges from 60 to 92 [19–23]. This high variability is likely due to the variable stage of disease and the mode of treatment. Lowest reported scoring scale among functioning scale is variable in literature: social functioning in Japan [21], role functioning in Sweden and China [19, 22] and emotional scale in Columbia and the USA [20, 23]. Due to inadequate infrastructure and limited expertise in minimally invasive approach to deal with cancer patients, open surgery is the main option for GI cancer surgery. This could be the reason of low physical functioning score, as majority of our patients underwent an open surgical procedure.
Among the nine symptom scales, financial impact was found to be the worst (42.94) followed by fatigue (30.21) and appetite loss (26.11). Sánchez et al [20] reported fatigue (44.5) as the worst parameter in gastric cancer and financial impact (36.3) as the worst parameter in colorectal cancer patients. As per Matsushita et al [21], sleep disturbance was with the worst score in symptoms scale. Fatigue was with the highest score in symptom scale as per Nordin et al [22]. The highest score with financial impact in our part was expected as majority of patients pay from their pocket and no healthcare uniformity exist for citizen from government. We are a private tertiary care hospital with state-of-the-art methods of treating cancer patients, so there is a selection bias and it may not be representative of all population. This is an area of very high importance and least discussed and needs further work. Many reforms are being introduced like heath cards which should improve this figure in near future.
Interpretation of the mean scores into meaningful clinical importance was a challenge. As majority of the work done was based on expert opinion, EORTC group systematically developed threshold for clinical importance (TCIs) after including the opinion of both patient and health professionals [13]. These thresholds have further increased the practicality of the questionnaire in real-life clinical scenarios. Table 3 describes the comparison of five functioning scales and nine symptom scales with TCI. Among functioning scales: Physical functioning was found to be below TCI while rest of the functioning scales were above TCI. Among symptom scales: financial impact and nausea/vomiting were two scales that were above TCI. We can appreciate that fatigue and appetite loss were ranked second and third in terms of worse symptom scale scores but their values were below TCI, and nausea/vomit ranked seventh but the score was found to be above TCI. So it is important to understand the value of the score and target to compare to in order to identify the areas where urgent focus is required to improve QOL in GI cancer patients. The reason for nausea/vomiting to be above TCI in GI cancer patients is not only due to the disease but also treatment modalities. Chemotherapy, radiation and surgery all affect GI motility.
Multiple factors affect the QOL in cancer patients including age, gender, marital status, employment status, level of education, nutritional status, presence of comorbidities, tumour location and stage of cancer [10, 11, 20, 23, 24]. In our study, patients who underwent surgery had a significantly better global QOL when compared with those who didn’t undergo any procedure. These findings are consistent with Calderón et al [26] and Amemiya et al [25]. who reported that patients with colorectal cancer and stomach cancer exhibited a better QOL post-surgery. This finding could be due to the fact that majority of our patients had colorectal cancers. Similar to existing literature, our study also showed that patients who are currently on treatment, i.e., adjuvant chemotherapy, had a poor global QOL. Chemotherapy, an essential component of cancer management comes with significant distress to patient at various stages of treatment plan [11, 27]. Multiple studies have demonstrated that presence of stoma is associated with poor QOL [28, 29]. Likewise our study shows that patients who had a stoma in their treatment plan had a significantly poor global QOL score. This finding was not surprising as having a stoma is a major concern in our population due to religious beliefs and adversely affects the QOL [30].
Functional capacity, documented as the Eastern Cooperative Oncology Group (ECOG) scale in our study, has a significant impact on QOL parameter. As per Schag et al [31], performance status is the best indicator of QOL. Likewise, in our analysis of all 15 scales combined, functional status of an individual was found to be a significant factor. History of previous surgery and higher grade of tumour were also found to be contributing to affect the QOL in our study but no such correlation is reported in available literature. Recurrence of tumour be it any site worsens the QOL of patients, studies have reported that even fear of recurrence has a significant impact on QOL of cancer patients [32, 33]. Multifactor model identified that those patients who had a recurrence has a significantly worse QOL and our findings are consistent with available literature [34, 35].
Conclusion
GI cancer symptoms and treatment plans vary as per the site of tumour, extending from oesophagus to the anal canal. Although EORTC have developed site-specific tools to assess QOL as per the site of tumour, we used EORTC QLQ C30 as its validated Urdu version was already available and few site-specific tools were in the process of translation into our native language. This is the first study from our region with the aim to develop baseline scores and factors severely affecting GI cancer patients in our community. A potential way forward would be to transform techniques to improve factors already identified, to plan multicentre studies representative of the whole population specially public sector and to perform site-specific studies using site-specific tools to establish means to improve QOL of our cancer patients in greater depth.
Conflicts of interest
No conflict of interest of any author.
Acknowledgements and funding
None.
References
1. Bray F, Laversanne M, and Weiderpass E, et al (2021) The ever-increasing importance of cancer as a leading cause of premature death worldwide Cancer 127(16) 3029–3030 https://doi.org/10.1002/cncr.33587 PMID: 34086348
2. Noronha V, Tsomo U, and Jamshed A, et al (2012) A fresh look at oncology facts on south central Asia and SAARC countries South Asian J Cancer 1(1) 1–4 [https://www.thieme-connect.com/products/ejournals/pdf/10.4103/2278-330X.96489.pdf] https://doi.org/10.4103/2278-330X.96489 PMID: 24455500 PMCID: 3876600
3. Xi L, Zhu J, and Zhang H, et al (2018) Epidemiological trends in gastrointestinal cancers in China: an ecological study Dig Dis Sci [Internet] 64(2) 532–543 https://doi.org/10.1007/s10620-018-5335-6 PMID: 30350242
4. Jansen L, Herrmann A, and Stegmaier C, et al (2011) Journal of Clinical Oncology health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study J Clin Oncol 29(24) 3263–3269 https://doi.org/10.1200/JCO.2010.31.4013 PMID: 21768465
5. WHOQOL Group (1993) Study protocol for the World Health Organization project to develop a quality of life assessment instrument (WHOQOL) Qual Life Res 2 153–159 https://doi.org/10.1007/BF00435734 PMID: 8518769
6. Aaronson NK, Ahmedzai S, and Bergman B, et al (1993) The European Organization for research and treatment for use in international clinical trials in oncology J Natl Cancer Inst 85(5) 365–376 https://doi.org/10.1093/jnci/85.5.365 PMID: 8433390
7. Velikova G, Coens C, and Efficace F, et al (2012) Health-related quality of life in EORTC clinical trials − 30 years of progress from methodological developments to making a real impact on oncology practice of life group and EORTC quality of life department Eur J Cancer Suppl [Internet] 10(1) 141–149 https://doi.org/10.1016/S1359-6349(12)70023-X
8. Weaver KE, Forsythe LP, and Reeve BB, et al (2012) Mental and physical health – related quality of life among US cancer survivors: population estimates from the 2010 national health interview survey Cancer Epidemiol Biomarkers Prev 21(11) 2108–2117 [https://aacrjournals.org/cebp/article/21/11/2108/158489/Mental-and-Physical-Health-Related-Quality-of-Life] https://doi.org/10.1158/1055-9965.EPI-12-0740 PMID: 23112268 PMCID: 3645867
9. Malik M, Rizwan I, and Hussain A (2021) Health related quality of life among blood cancer patients in Pakistan: a cross sectional survey Inquiry 58 00469580211025211 PMID: 34130537 PMCID: 8212374
10. Saleha SB, Shakeel A, and Shumaila E, et al (2010) An assessment of quality of life in breast cancer patients using EORTC QLQ C30/+ Br23 questionnaire Int J Cancer Manag 3(2) e80684 [https://brieflands.com/articles/ijcm-80684.html]
11. Chagani P, Parpio Y, and Gul R, et al (2017) Quality of life and its determinants in adult cancer patients undergoing chemotherapy treatment in Pakistan Asia Pac J Oncol Nurs 4(2) 140 https://doi.org/10.4103/2347-5625.204499 PMID: 28503647 PMCID: 5412152
12. Fayers P, Aaronson N, and Bjordal K, et al (2001) EORTC QLQ-C30 Scoring Manual The EORTC QLQ-C30 Introduction. EORTC QLQ-C30 Scoring Mannual [Internet] vol 30 (Brussels: EORTC) pp 1–67 [https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf]
13. Giesinger JM, Loth FLC, and Aaronson NK, et al (2020) Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research J Clin Epidemiol [Internet] 118 1–8 https://doi.org/10.1016/j.jclinepi.2019.10.003
14. Ihn MH, Lee SM, and Son IT, et al (2015) Cultural adaptation and validation of the Korean version of the EORTC QLQ-CR29 in patients with colorectal cancer Supportive Care Cancer 23 3493–3501 https://doi.org/10.1007/s00520-015-2710-0
15. Shen MH, Chen LP, and Ho TF, et al (2018) Validation of the Taiwan Chinese version of the EORTC QLQ-CR29 to assess quality of life in colorectal cancer patients BMC Cancer 18 353 https://doi.org/10.1186/s12885-018-4312-y PMID: 29606101 PMCID: 5880067
16. Cerezo O, Oñate‐Ocaña LF, and Arrieta‐Joffe P, et al (2012) Validation of the Mexican‐Spanish version of the EORTC QLQ‐C30 and BR23 questionnaires to assess health‐related quality of life in Mexican women with breast cancer Eur J Cancer Care 21(5) 684–691 https://doi.org/10.1111/j.1365-2354.2012.01336.x
17. Sadighi S, Montazeri A, and Sedighi Z, et al (2009) Quality of life in patients with gastric cancer: translation and psychometric evaluation of the Iranian version of EORTC QLQ-STO22 BMC Cancer 9(1) 1–8 https://doi.org/10.1186/1471-2407-9-305
18. Flechtner H and Bottomley A (2006) Quality of life assessment and research in the EORTC (European Organisation for Research and Treatment of Cancer) Oncologie 8(5) 443–446 https://doi.org/10.1007/s10269-006-0412-4
19. Hong Y, Wu C, and Wu B (2020) Effects of resistance exercise on symptoms, physical function, and quality of life in gastrointestinal cancer patients undergoing chemotherapy Integr Cancer Ther 19(248) 1534735420954912 https://doi.org/10.1177/1534735420954912 PMID: 32909468 PMCID: 7493268
20. Sánchez R, Alexander-Sierra F, and Oliveros R (2012) Relationship between quality of life and clinical status in patients with gastrointestinal cancer Rev Esp Enfermedades Dig 104(11) 584–591 https://doi.org/10.4321/S1130-01082012001100006
21. Matsushita T, Matsushima E, and Maruyama M (2004) Assessment of peri-operative quality of life in patients undergoing surgery for gastrointestinal cancer Support Care Cancer 12(5) 319–325 https://doi.org/10.1007/s00520-004-0618-1 PMID: 15064934
22. Nordin K, Steel J, and Hoffman K, et al (2001) Alternative methods of interpreting quality of life data in advanced gastrointestinal cancer patients Br J Cancer 85(9) 1265–1272 https://doi.org/10.1054/bjoc.2001.2046 PMID: 11720459 PMCID: 2375246
23. Velasco Jr RN, Catedral LI, and Chua Jr AV, et al (2022) The impact of malnutrition on the quality of ife of colorectal cancer patients in a tertiary hospital Nutr Cancer 18 1–9 https://doi.org/10.1080/01635581.2022.2044061 PMID: 35225108
24. Heydarnejad MS, Hassanpour DA, and Solati DK (2011) Factors affecting quality of life in cancer patients undergoing chemotherapy Afr Health Sci 11(2) PMID: 21857860 PMCID: 3158510
25. Amemiya T, Oda K, and Ando M, et al (2007) Activities of daily living and quality of life of elderly patients after elective surgery for gastric and colorectal cancers Ann Surg 246(2) 222–228 [https://journals.lww.com/annalsofsurgery/Abstract/2007/08000/Activities_of_Daily_Living_and_Quality_of_Life_of.10.aspx] https://doi.org/10.1097/SLA.0b013e3180caa3fb PMID: 17667500 PMCID: 1933572
26. Calderón C, Jiménez-Fonseca P, and Hernández R, et al (2019) Quality of life, coping, and psychological and physical symptoms after surgery for non-metastatic digestive tract cancer Surg Oncol [Internet] 31 26–32 https://doi.org/10.1016/j.suronc.2019.08.009 PMID: 31493647
27. Mo B, Yoon K, and Lee S, et al (2020) Quality of life and patient satisfaction after single ‑ and multiport laparoscopic surgery in colon cancer: a multicentre randomised controlled trial (SIMPLE Trial) Surg Endosc [Internet] 35 6278–6290 https://doi.org/10.1007/s00464-020-08128-9
28. Faury S, Rullier E, and Denost Q, et al (2020) Quality of life and fatigue among colorectal cancer survivors according to stoma status – the national VICAN survey survivors according to stoma status – the national J Psychosoc Oncol [Internet] 38(1) 89–102 https://doi.org/10.1080/07347332.2019.1638481
29. De Almeida Silva K, Xavier Duarte A, and Rodrigues Cruz A, et al (2020) Ostomy time and nutrition status were associated on quality of life in patients with colorectal cancer J Coloproctol 40(4) 352–361 https://doi.org/10.1016/j.jcol.2020.07.003
30. Ahmad M, Jamal S, and Ahmad N (2011) Quality of life Assessment in patients with stoma in muslim population Ann Pak Inst Med Sci 7(4) 222–227 [http://apims.net/apims_old/Volumes/Vol7-4/QUALITY%20OF%20LIFE%20ASSESSMENT%20IN%20PATIENTS%20WITH%20STOMA%20IN%20MUSLIM%20POPULATION.pdf]
31. Schag CAC, Ganz PA, and Wing DS, et al (1994) Quality of life in adult survivors of lung, colon and prostate cancer Qual Life Res 3(2) 127–141 https://doi.org/10.1007/BF00435256 PMID: 8044158
32. Choi JH, Kim ES, and Lee YJ, et al (2015) Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer Gastrointest Endosc [Internet] 82(2) 299–307 https://doi.org/10.1016/j.gie.2015.01.019 PMID: 25892060
33. Chen WY, Wang S, and Peng X, et al (2021) Trait emotional intelligence and quality of life among breast cancer patients: the mediating role of fear of cancer recurrence Int J Nurs Pract 28(4) 1–9 https://doi.org/10.1111/ijn.12953
34. Glyn T and Frizelle F (2020) Quality of life outcomes in patients undergoing surgery for locally recurrent rectal cancer Semin Colon Rectal Surg [Internet] 31(3) 100767 https://doi.org/10.1016/j.scrs.2020.100767
35. Camilleri-Brennan J and Steele RJC (2001) The impact of recurrent rectal cancer on quality of life Eur J Surg Oncol 27(4) 349–353 https://doi.org/10.1053/ejso.2001.1115 PMID: 11417978






