Improvement of diagnosis in children with Burkitt lymphoma in Kenya: feasibility study for the implementation of fluorescence in situ hybridisation testing for MYC and the MYC/IGH translocation
Gail H Vance1, Teresa Lotodo2, Nicholas Kigen3, Ryan Stohler1, Haki Choi1, Festus Njuguna4, Ann M Moormann5, Erastus Kirwa6, Sandra Langat7, Patrick Loehrer8 and Terry Vik9
1Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN 46202, USA
2Department of Pathology, Moi University, Eldoret, Kenya
3AMPATH Reference Laboratory, Eldoret, Kenya
4Department of Child Health and Pediatrics, Moi University, Eldoret, Kenya
5Department of Medicine, University of Massachusetts Chan Medical School, Worcester, MA 01655, USA
6Kenya Medical Research Institute (KEMRI), Kisumu, Kenya
7AMPATH Pediatric Oncology, Eldoret, Kenya
8Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202, USA
9Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN 46202, USA
Abstract
Background: Indiana University (IU) initiated fluorescence in situ hybridisation (FISH) methodology for Burkitt Lymphoma (BL) to advance the accuracy and speed of diagnosis in the AMPATH Reference Laboratory at Moi Teaching and Referral Hospital (MTRH) in Eldoret, Kenya. Standard diagnostic testing for BL at MTRH includes morphology of the biopsy specimen or aspirate and limited immunohistochemistry panels.
Methods: Tumour specimens from 19 children enrolled from 2016 to 2018 in a prospective study to improve the diagnosis and staging of children with suspected BL were evaluated. Touch preps from biopsy specimens or smears from fine needle aspiration were collected, stained with Giemsa and/or H&E and reviewed by pathologists to render a provisional diagnosis. Unstained slides were stored and later processed for FISH. Duplicate slides were split between two laboratories for analysis. Flow cytometry results were available for all specimens. Results from the newly established FISH laboratory in Eldoret, Kenya were cross-validated in Indianapolis, Indiana.
Results: Concordance studies found 18 of 19 (95%) of specimens studied yielded analysable FISH results for one or both probe sets (MYC and MYC/IGH) in both locations. There was 94% (17/18) concordance of results between the two FISH laboratories. FISH results were 100% concordant for the 16 specimens with a histopathological diagnosis of BL and two of three non-BL cases (one case no result in IU FISH lab). FISH was similarly concordant with flow cytometry for specimens with positive flow results with the exception of a nasopharyngeal tumour with positive flow results for CD10 and CD20 but was negative by FISH. The modal turn-around time for FISH testing on retrospective study specimens performed in Kenya ranged between 24 and 72 hours.
Conclusion: FISH testing was established, and a pilot study performed, to assess the feasibility of FISH as a diagnostic tool for the determination of BL in a Kenyan paediatric population. This study supports FISH in limited resource settings to improve the accuracy and speed of diagnosis of BL in Africa.
Keywords: Burkitt lymphoma, fluorescence in situ hybridisation, FISH, MYC/IGH translocation, MYC rearrangement
Correspondence to: Gail H Vance
Email: ghvance@iu.edu
Published: 08/02/2023
Received: 15/11/2022
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Burkitt lymphoma (BL) is a common paediatric cancer representing greater than 50% of childhood cancers in sub-Saharan Africa. A subtype of non-Hodgkin lymphoma (non-HL), endemic BL (eBL) is associated with Epstein–Barr virus and continued exposure to Plasmodium falciparum malaria infections [1, 2]. A highly aggressive tumour, delay of diagnosis results in delay of treatment, resulting in high morbidity and mortality [3–5].
Fluorescence in situ hybridisation (FISH) is a molecular cytogenetic tool for identifying recurring translocations in haematological malignancies [6, 7]. In BL, the t(8;14)(q24;q32) is the characteristic cytogenetic translocation involving the juxtaposition of MYC with IGH on chromosome 14 resulting in MYC dysregulation [8, 9]. Probes flanking the MYC and IGH breakpoints create a dual (or tri) colour, dual fusion signal pattern in BL cells containing the translocation. Disruption of the MYC gene locus may also be identified with a break apart probe strategy.
This FISH diagnostic pilot study was initiated in the AMPATH Reference Laboratory (ARL) at Moi Teaching and Referral Hospital (MTRH) in Eldoret, Kenya and supported by an Administrative Supplement (PA-16-086) to an active clinical trial with the aim to complement and improve histological diagnosis of a core needle biopsy or fine needle aspiration (FNA) of the tumour. Eligibility for the clinical trial included a clinical suspicion of BL in a child 0–13 years of age. Children with prior treatment of cancer were excluded. The trial was approved by Institutional Review Boards of Indiana University (IU) and MTRH. Parental consent was obtained prior to study entry.
Materials and methods
From 2016 through 2018, 96 children of 0–13 years with clinical suspicion of BL were recruited at MTRH in Eldoret, Kenya. A tissue biopsy or FNA, or both, was obtained from the child’s tumour. Tissue was collected from various MTRH locations including the surgical suite, radiology, dental services and others. The biopsy tissue was generally divided into two pieces with one piece placed in formalin for histological diagnosis and another biopsy core was touched to positively charged slide(s) and taken to the ARL. FNA aspirates were prepared in the procedure room. Aspirate material was smeared on slides for histological review and also deposited into Roswell Park Memorial Institute (RPMI) media for flow cytometry. FNA smears were stained with Giemsa and/or H&E and reviewed by pathologists to render a histopathological diagnosis. Flow cytometry was performed on a BD FACS Calibur in ARL. The sample was subjected to a leukaemia/lymphoma panel that included CD10, CD19, CD20, kappa and lambda. For the purposes of this study, a value of ≥20% for CD10, CD19 or CD20 was considered significant. Additional unstained slides, when available, were stored for future studies.
A total of 96 children were enrolled and 43 cases of BL were confirmed by histopathology on biopsy specimens (Figure 1). Of materials collected, initially for flow, then FISH, FISH required two slides for testing in the two FISH laboratories. Concordance studies were performed between the ARL and IU Cytogenetics FISH laboratories. Stored slides representing 19 patient tumours were interrogated by FISH to assess feasibility and accuracy of testing. Cases with duplicate unstained slides from either a tissue biopsy touch prep or FNA were used for FISH and split between the two institutions. Slides had been stored in slide boxes, exposed to ambient air temperature prior to FISH testing. Once the study slides were selected, they were immersed in 3:1 methanol:acetic acid fixative for 20 minutes and air-dried. Slides processed in the IU FISH laboratory were transported from Eldoret in plastic slide carriers and placed in checked luggage until arrival in the FISH laboratory and immediately immersed in fixative.
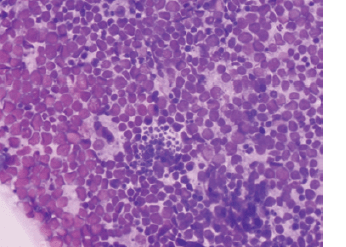
Figure 1. Tumour from MBL-019: abdominal mass, core biopsies. Sections show a tumour composed of sheets of intermediate cells with scant cytoplasm, irregular hyperchromatic nuclei, inconspicuous nucleoli and brisk mitotic activity. Focal area exhibits a ‘starry sky’ appearance. Morphologic features consistent with BL.
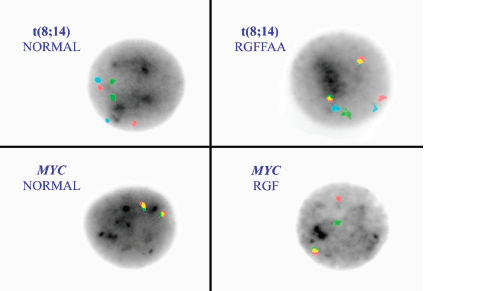
Figure 2. FISH on interphase nuclei with the t(8;14) and MYC break apart probes. The left upper corner demonstrates a normal FISH pattern (left) for the t(8:14) with RRGGAA. Red = MYC, Green = IGH and aqua (A) is the chromosome 8 centromere. The right upper corner is the abnormal dual fusion signal pattern representing a t(8;14). The lower panels show a normal MYC break apart probe pattern (fusion/fusion signal pattern) on the left and a rearrangement pattern RGF on the right.
The most common cytogenetic translocation associated with BL and found in approximately 85% of the BL is the t(8;14) involving the MYC gene on chromosome 8 and the IGH gene on chromosome 14. Probes for MYC and IGH were utilised. The tri-colour, dual fusion probe set (Figure 2) included probes that hybridise to MYC (red), IGH (green) and the chromosome 8 centromere (aqua) (LSI IGH/MYC/CEP 8 Tri-color Dual Fusion Probe Kit-Abbott Molecular, Des Plaines, IL). There are also two variant BL translocations, t(2;8)(p12;q24) and t(8;22)(q24;q11.2) involving IGK (15%) and IGL (5%) of BL cases, respectively. Therefore, we also incorporated a MYC break apart strategy (LSI MYC Dual Color Break Apart Rearrangement Probe-Abbott Molecular) to confirm the t(8;14) and potentially detect variant translocations (Figure 2) in the cases with negative t(8;14) results but positive by flow cytometry and with a histopathological diagnosis of BL. The MYC probe is disrupted with a rearrangement of MYC to any partner locus; 5’ segment of MYC (red) and 3’ segment (green) split to yield a red/green/fusion (RGF) rearrangement pattern.
Interphase FISH was performed following a standard, shared protocol using direct-labelled probes. Briefly, unstained touch prep and FNA slides were fixed in 3:1 methanol:acetic acid and allowed to air dry. They were pre-treated in 2× saline sodium citrate buffer (SSC) followed by 15 minutes of 0.005% pepsin, both at 37°C. Denaturation, ethanol dehydration and hybridisation with the LSI MYC and LSI IGH/MYC, CEP 8 probes were carried out as per manufacturer’s instructions. Cells were counterstained with DAPI (4’,6,diamidino-2-phenylindole) and observed under a Leica DM2000 LED fluorescence microscope. One hundred cells scored for each probe set at the respective laboratory.
A haematopathologist (TL) and one lab scientist (NK) spent 2 weeks in the IU Cytogenetics FISH laboratory training on FISH protocols, learning FISH microscopy and FISH result interpretation. The IU Cytogenetics FISH laboratory is Clinical Laboratory Improvement Amendments (CLIA)-certified and accredited by the College of American Pathologists. The Leica fluorescence microscope with appropriate filters and imaging capabilities was purchased in the U.S. and established in laboratory space donated by the ARL in 2017, midway during the clinical trial. FISH probes were brought from the US and other reagents purchased via the AMPATH research office. A study investigator (GHV) travelled to Eldoret to oversee initial lab setup and develop the IU Cytogenetics FISH testing protocols in the ARL.
Results
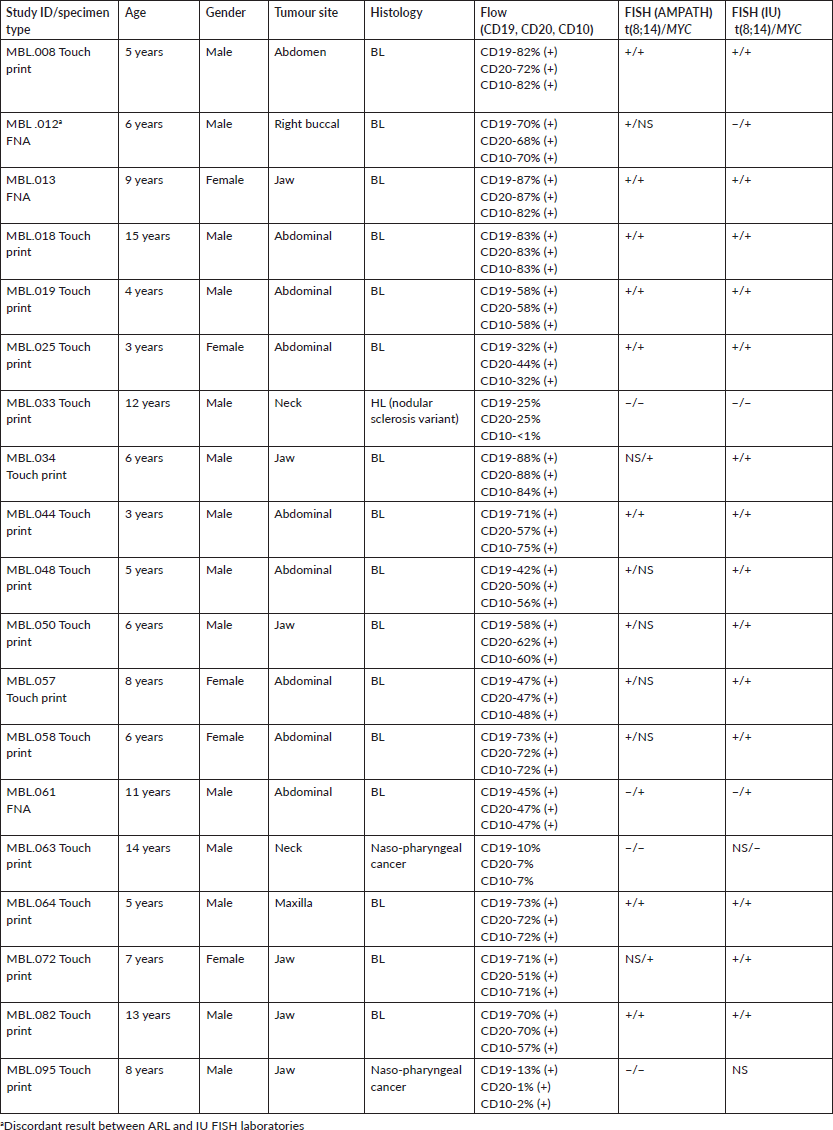
Nineteen specimens were studied in the AMPATH and IU FISH laboratories. Results for one or two probe sets were obtained in both laboratories for 18/19 specimens (95%) (Table 1). Concordant results occurred in 17 of 18 cases (94%) with a disagreement of the t(8;14) result for study specimen MBL12. A t(8;14) signal pattern was identified in the ARL but not in the IU FISH laboratory although the IU lab did detect a MYC rearrangement. There were no results for MYC from the ARL for this specimen and additional slides to repeat the testing were not available.
Sixteen of the 19 specimens had a histological diagnosis of BL (Table 2). The three non-BL tumours included MBL-33, MBL-63 and MBL-95 classified as nodular variant of HL (MBL-33) and nasopharyngeal cancer (MBL-63, MBL-95). FISH results from both laboratories were positive/concordant for the t(8;14) or MYC rearrangement in all 16 histological cases of BL cases and negative for either the t(8;14) and/or MYC probe in the three non-BL cases although the IU lab had indeterminate results for both probes for specimen MBL-095 and no signal (NS) for the t(8;14) for MBL-63.
The 16 BL specimens also had positive flow results indicating concordance with histological diagnosis, flow cytometry and FISH for these specimens. Flow cytometry results for CD19 and CD20 were also positive for MBL-33, the nodular lymphocyte-predominant variant of HL (NLPHL).
Specimen MBL-061 was positive for MYC rearrangement only in both labs indicating it may represent a variant BL t(2;8) or t(8;22) or another aggressive lymphoma with MYC rearrangement. Five specimens (MBL-012, 048, 050, 057, 058) studied in the AMPATH FISH laboratory were unsuccessful for MYC detection although a MYC rearrangement was detected on the same specimens in the IU FISH laboratory. It was determined that the placement of the MYC probe was too far to the right of the optimal cellular area for successful microscopic FISH analysis. Subsequent adjustment was made to load both probe sets closer to the frosted edge of the slide and to mark the slide for probe placement based on a microscopic pre-screen of the slide. Additional slides for these tumours were not available for re-analysis of MYC.
Table 1. Summary of FISH study results.
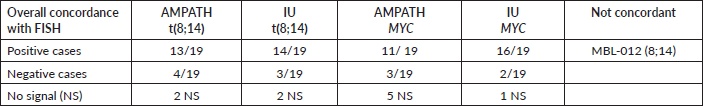
Table 2. Study subjects: clinical information and laboratory results.
Discussion
FISH is a powerful diagnostic tool for screening, early detection, tumour classification and monitoring the efficacy of interventions in leukaemia and lymphoma [8, 10]. Metaphase preparations from cultured cells are often considered the ‘gold standard’ because chromosome morphology and position of the fluorescent signals may be visualised directly. However, a major advantage of FISH is that it can also be performed on non-dividing interphase cells. Interphase nucleus assessment from uncultured preparations allows for a rapid screening for specific chromosome rearrangements or numerical abnormalities associated with haematologic malignancies [7].
In this pilot study, a FISH laboratory was established in Kenya and interphase FISH testing was introduced on uncultured biopsy touch prep specimens and FNA smears from suspected BL patients enrolled in a clinical trial. Concordance studies were performed between the ARL and IU Cytogenetics FISH laboratories utilising probe sets for the t(8;14) and MYC.
Eighteen of the 19 (95%) specimens studied yielded analysable FISH results for one or both probe sets in both locations. The IU FISH lab was unable to obtain results for either probe set on MBL-095. There was 94% (17/18) concordance of results between the two FISH laboratories demonstrating the feasibility of performing FISH for BL in the ARL. Further, we demonstrate concordance of FISH with a histological diagnosis of BL and positive flow cytometry results. One non-Burkitt specimen (MBL-33) had positive flow results for CD19 and CD20. This specimen was determined to be a NLPHL. NLPHL is a morphologically related subtype, but clinically and pathogenetically distinct from classic HL with large and atypical lymphocyte-predominant cells that express B-cell markers such as CD20+ [11, 12].
To improve the accuracy of FISH in diagnosis of BL, two probe strategies were utilised, a tri-colour, dual fusion strategy for the t(8;14) and a break apart strategy for MYC. For the 16 specimens with a histological diagnosis of BL, positive results with the t(8;14) probe set were obtained for 13/16 (81%) in the ARL lab compared to 14/16 (87.5%) in the IU FISH lab. Both labs showed negative results for the translocation for MBL-61 with Burkitt histology and positive MYC FISH results. This specimen was thought to represent a variant BL translocation. There were no results for the t(8;14) in specimens MBL-034 and MBL-072 in the ARL lab and the t(8;14) was negative for MBL-012 in the IU FISH lab, yielding discordant results with the ARL lab FISH, histological diagnosis and flow results. The IU FISH lab had 100% concordance with histological diagnosis and flow results for the MYC probe and the 16 BL specimens compared to 69% for the ARL lab primarily due to laboratory error in probe placement. The IU FISH laboratory had no results for either the t(8;14) or MYC probe for specimen MBL-95. The quality of this specimen may have been affected by prolonged storage and shipment to Indianapolis.
Noting that positive MYC FISH results alone are not sufficient to support a diagnosis of BL, Troxell et al [13] reported on seven clinically or morphologically suspicious cases for BL. Six of seven were positive for MYC rearrangement by FISH but only 3/6 represented BL with one designated as an atypical BL. The study illustrated the value of FNA in diagnosis of BL as well as the importance of integrating results of ancillary studies noting that MYC may be rearranged in other aggressive lymphomas. A similar study by Chen et al [14], looking at clinical pathological analysis of B-cell lymphomas concluded that the use of two probe sets with MYC rearrangement and MYC/IGH fusion was detected in 94.2% (81/86) and 83.7% (72/86) cases of BL. Our study, like that of Chen et al [14], utilised the MYC rearrangement probe and the probe set for the typical BL translocation. We also included flow cytometry with a panel of antibodies for recognition of B-cell neoplasia thus supporting the sensitivity and accuracy of our FISH results for BL.
FISH testing was also undertaken to improve the speed of BL diagnosis. Njuguna et al [15] studied the time lag and other factors influencing the time to diagnosis and start of treatment among paediatric oncology patients in Kenya. For that study, participants were enrolled at MTRH from August 2013 to July 2014. Diagnostic delay was defined as the time from the onset of symptoms to diagnosis. The time of diagnosis from arrival at MTRH was reported to be approximately 11 days. For this current study conducted from November 2016 to March 2018, specimens were retrieved from several MTRH locations and prepared for flow cytometry and FISH. Once received in the ARL FISH laboratory, FISH results with the MYC and MYC/IGH probe sets were available within 24–72 hours, similar to FISH testing turnaround times in other international laboratories.
A limitation of our study was the identification of two or more unstained slides available for FISH in both laboratories reducing the total number of study specimens. Further, the slides were stored in ambient temperature and not fixed with a methanol/acetic acid solution after collection allowing for degradation of the cells. A variant BL translocation including a t(2;8) or t(8;22) was suspected for MBL-061; however, further definitive testing for these translocations was not included in our study protocol.
Conclusion
Here we demonstrate the development of FISH as a diagnostic tool for BL in Eldoret, Kenya. To the authors’ knowledge, FISH testing in the ARL is the only FISH testing performed for cancer in Kenya. The study achieved the objectives of establishing a FISH laboratory and training personnel for successful performance of FISH testing, thus reducing the diagnostic interval from tissue procurement to BL diagnosis. Introducing FISH for a more conclusive diagnosis of BL and other haematological disorders in an under-resourced public health facility is a major milestone. It shows that major strides are being made to include ancillary tests like immunophenotyping, cytogenetics and molecular tests in diagnosis of haematological disorders as recommended by the World Health Organization.
Our study to assess the utility of FISH to improve time to diagnosis of eBL in this population was a feasibility study to test whether it was even possible to use this test in a low and middle income country (LMIC). FISH testing performed in this study was supported by grants and academic funds. Going forward, it would be important for the Kenyan National Health Insurance Fund to consider coverage for testing. We plan to introduce FISH into standard clinical study protocols and to assess potential changes in the clinical outcomes for the study participants including reducing time from symptom onset to diagnosis and initiation of therapy.
Authors’ contributions
Conceptualisation and study design: GHV, TL, FN, AMM, PL, TV
Technical work and data collection: GHV, TL, NK, RS, HC, EK, TV
Data management: GHV, TL, NK, RS, HC, SL
Writing: GHV, TL, AMM, TV
All authors read and approved the manuscript in its current form.
Acknowledgments and funding
This work was supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) as a supplement (3 P30 CA082709-18S3) to the Indiana University Melvin and Bren Simon Comprehensive Cancer Center study titled ‘Improvements of diagnosis, staging and support of children with Burkitt lymphoma’ (P. Loehrer, PI). Dr Vance is supported by the Sutphin family professorship and fund. The authors are grateful for the scientific and technical support of the programme provided by Takeda Pharmaceutical and they especially thank the parents and the children for participating in this study.
Conflicts of interest
The authors declare that they have no competing interests.
References
1. Moormann A and Bailey J (2016) Malaria-how this parasitic infection aids and abets EBV-associated Burkitt lymphomagenesis Curr Opin Virol 20 78–84 https://doi.org/10.1016/j.coviro.2016.09.006 PMID: 27689909 PMCID: 5102755
2. Bouda GC, Traore F, and Couitchere L, et al (2019) Advanced Burkitt lymphoma in sub-Saharan Africa pediatric units: results of the third prospective multicenter study of the Groupe Franco-Africain d’oncologic pediatrique J Glob Oncol 5 1–9 PMID: 31794283 PMCID: 6939747
3. Aka P, Kawira E, and Masalu N, et al (2012) Incidence and trends in Burkitt lymphoma in Northern Tanzania from 2000 to 2009 Pediatr Blood Cancer 59 1234–1238 https://doi.org/10.1002/pbc.24194 PMID: 22618958 PMCID: 3427713
4. Mosert S, Njuguna F, and Kempa L, et al (2012) Epidemiology of diagnosed childhood cancer in Western Kenya Arch Dis Child 97 508–512 https://doi.org/10.1136/archdischild-2011-300829
5. Martijn HA, Njuguna F, and Olbara G, et al (2017) Influence of health insurance status on paediatric non-Hodgkin’s lymphoma treatment in Kenya BMJ Paediatr Open 1(1) e000149 https://doi.org/10.1136/bmjpo-2017-000149
6. Wolff DJ, Bagg A, and Cooley LD, et al (2007) Guidance for fluorescence in situ hybridization testing in hematologic disorders J Mol Diagn 9(2) 134–143 https://doi.org/10.2353/jmoldx.2007.060128 PMID: 17384204 PMCID: 1867444
7. Vance GH and Khan WA (2021) Utility of fluorescence in situ hybridization in clinical and research applications Adv Mol Pathol 3 65–75 https://doi.org/10.1016/j.yamp.2020.07.006
8. Aquino G, Marra L, and Cantile M, et al (2013) MYC chromosomal aberration in differential diagnosis between Burkitt and other aggressive lymphomas Infect Agents Cancer 8(1) 37 https://doi.org/10.1186/1750-9378-8-37
9. Ferry JA (2006) Burkitt’s lymphoma: clinicopathologic features and differential diagnosis Oncologist 11(4) 375–383 https://doi.org/10.1634/theoncologist.11-4-375 PMID: 16614233
10. Gerhard-Hartmann E and Rosenwald A (2020) Fluorescence in situ hybridization in the diagnosis of aggressive B-cell lymphomas Pathologe 41(6) 574–581 https://doi.org/10.1007/s00292-020-00816-6 PMID: 32909092
11. Schani AR, Jaffe ES, and Harris NL, et al (2011) Nodular lymphocyte-predominant hodgkin lymphoma with atypical T cells: a morphologic variant mimicking peripheral T-cell lymphoma Am J Surg Pathol 35(11) 1666–1678 https://doi.org/10.1097/PAS.0b013e31822832de
12. Grewal BK, Chetty M, and Abayomi EA, et al (2019) Use of flow cytometry in the phenotypic diagnosis of hodgkin’s lymphoma Clin Cytometry 96B 116–127 https://doi.org/10.1002/cyto.b.21724
13. Troxell ML, Bangs CD, and Cherry AM, et al (2005) Cytologic diagnosis of Burkitt lymphoma Cancer Cytopathol 105(5) 310–318 https://doi.org/10.1002/cncr.21307
14. Chen M, Yang JL, and Zhao S, et al (2018) Diagnostic and therapeutic values of interphase fluorescence in situ hybridization in B-cell lymphomas: a clinicopathologic analysis of 604 cases Zhonghua Bing Li Xue Za Zhi 47(12) 920–925 PMID: 30522172
15. Njuguna F, Martijin H, and Langat S, et al (2016) Factors influencing time to diagnosis and treatment among pediatric oncology patients in Kenya Pediatr Hematol Oncol 33(3) 186–199 https://doi.org/10.3109/08880018.2016.1169566 PMID: 27184775