Review of the literature on combined oral contraceptives and cancer
Mustafa Kamani, Utku Akgor and Murat Gültekin
Department of Obstetrics and Gynaecology, Hacettepe University Faculty of Medicine, Ankara 06230, Turkey
Abstract
Millions of women have given preference to the use of combined oral contraceptives (COCs) since its introduction in the 1960s. Both oestrogens and progestogens can regulate proliferation and it is plausible these effects may contribute to carcinogenesis. We aimed to review the accumulated knowledge to date to appreciate the modifying effects combined oral contraceptives may have on carcinogenesis. Our methodology involved a review of the current published literature, paying attention to studies published in the last 20 years. It has been noted that the overall cancer odds do not change with the use of COCs. Increased risk for breast cancer with COC use is not consistently backed in the literature; the results range from no increase in risk to a 20%–30% elevation in risk, and the risk seems to be temporary, limited to recent or current regular COC use. Also, diagnosed breast cancer cases seem to be clinically advanced in ever-users compared to never-users. Data show that the ongoing and prolonged use of COCs may provide diminished risk for endometrial, colorectal and ovarian cancers. Although studies do not clearly support increased risk with COC use in high-risk groups, such as women with family history of cancer or BRCA carriers, local and international guidelines are available for clinical decision-making. For cervical cancer, COCs seem to enhance the risk with more than 5 years of use, and in many studies, this enhanced risk diminishes after discontinuation and restores to those of never-users within 10 years. The relationship between COC use and liver malignancy risk assessments has provided conflicting findings. Some studies have suggested that hormonal contraceptives may increase the risk of not only hepatocellular carcinoma but also intrahepatic cholangiocarcinoma. Combined oral contraceptives are safe and effective and the effects are reversible. Patients who pursue family planning should be warned of possible carcinogenic outcomes, but it should also be explained that—in addition to sexual health advantages—preferring COCs may also decrease the risks of endometrial, colorectal and ovarian cancers.
Keywords: combined contraceptives, hormonal contraception, breast cancer, ovarian cancer, cervical cancer, liver cancer
Correspondence to: Mustafa Onur Kamani
Email: Onurkamani@gmail.com
Published: 23/06/2022
Received: 09/11/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Combined oral contraceptives are classified by the World Health Organisation among Group 1 carcinogens for their possible relationship to liver cancer, in situ and invasive cancer of the uterine cervix and breast cancer [1]. From the time of its addition to the contraceptive arsenal, millions of women have used COCs, oftentimes for lengthy durations and at a period of well-being. Since the development and approval of the first COC pill, formulations have evolved from comprising high-dose oestrogen (150 mcg) to very low doses (10 and 20 mcg). Although there is a wide array to choose from, and there are many doses accessible, all consist of a progestogen and an oestrogen. Pregnancy is averted fundamentally via blocked ovulation. In addition, alterations to the endometrium and cervical mucus are also important factors preventing pregnancy [2]. The users were especially concerned about cancer, given the widespread usage and frequent media scares. Both oestrogens and progestogens can regulate cell growth and reproduction, and it is plausible that this proliferative influence may contribute to carcinogenesis in susceptible tissues. The aim of this review was to analyse the available up-to-date literature regarding malignancy risks linked to the use of COCs. The search was performed in the PubMed database for oral contraceptive/combined oral contraceptives/contraceptives/contraception in title or abstract and cancer in title. A total of 2,483 papers were returned. Titles of the last 20 years were the primary focus and studies published in high-impact journals, which have large cohort sizes and high citation counts, were selected for more in-depth narrative reviews.
Overall cancer risk
Up to now, there have not been any studies showing that overall cancer risk or mortality related to cancer in general has increased among COC users. Cohort studies are notably advantageous when it comes to analysing the general proportion of risks and advantages related to a COC exposure. Absolute risk of mortality from cancer was assessed in COC users in three large cohort studies, which are the Royal College of General Practitioners (RCGP) study, the Nurses’ Health Study and the Oxford Family Planning Association research; between ever-users and never-users, none of these studies found significant discrepancy [3–5]. Overall, cancer possibility does not increase with COC use. The evidence proposes that ongoing users of COCs may have elevated odds of malignancy of the liver, cervix and breast in comparison with non-users. The majority of studies propose that the elevated risks of mentioned malignancies deteriorate subsequently after cessation of COCs, restoring within about 10 years to that of non-users. On the other hand, ongoing and prolonged use of COCs provide diminished risks for ovarian, endometrial and probably colorectal cancers. This protection appears to remain for years after cessation of COCs, as it may exceed 30 years. Long-standing cancer benefits might neutralise the interim adverse effects on the assumption that they persevere until later in life because the majority of malignancies become frequent after 50 years of age.
Long-term malignancy risks and benefits were analysed in the RCGP oral hormonal pregnancy prevention study, which covered a cohort of more than 46,000 women who were followed up as far as 44 years [6]. In this study, it is reported that patients who had used COCs are not in danger of new malignancy risks later in life. Regular COC use may have a protective effect from ovarian, colorectal and endometrial malignancies, which are likely to continue many years after cessation, possibly more than 35 years in the cases of ovarian and colorectal cancer. Enhanced risks for cancer of the breast and cancer of the uterine cervix that are observed in recent and ongoing users are stated to be temporary, diminishing after 5 years of cessation to those of never-users.
Long-term cancer risks were evaluated in a cohort of 267,400 female textile workers in China; no increased risk of cancers combined or of thyroid, liver, breast, lung, ovarian, colon, pancreatic, gall bladder, rectal, cervical or stomach cancers were detected. Only cancers of the uterine corpus were found to be reduced with COC use [7].
Breast cancer
Oestrogens and progestogens are among the modifiable factors that may increase the risk of breast cancer (BC) and this has been confirmed, for instance, in hormone replacement treatment (HRT) in the post-menopausal population. From the time of its introduction, a similar proposal has been suggested for COCs. In the breast, both oestrogen and progesterone have a proliferating effect, probably due to stem cell stimulation [8]. Inherent oestrogens (oestradiol and oestrone) are suggested mutagens over carcinogens by way of a gene damaging mechanism [9], whereas the mediating route of progestin is more complicated [10, 11] (Figure 1b). Scepticism remains regarding the association between the consumption of COCs and the risk of BC. Researches of BC risk among women who use COCs show conflicting results: from no increase in risk to a 20%–30% elevation in risk. The majority of articles have categorised participants in accordance to whether they were ongoing, recent or previous users of COCs or if they had ever used COCs. The findings of many epidemiologic studies state no association. Any effect seems to be temporary or limited to recent or ongoing COC usage (Table 1).

Figure 1. Pathways correlating the effect of COCs on breast cancer.
Table 1. Studies reporting no increase in the risk of breast cancer associated with COC use.
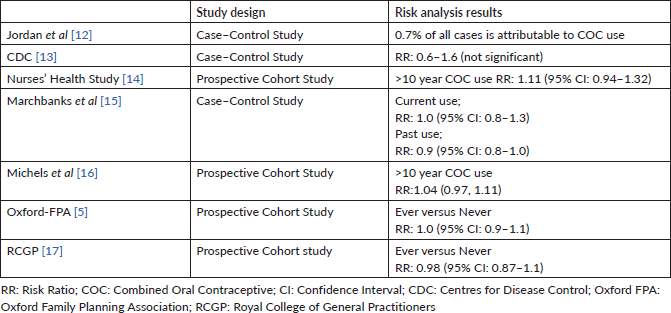
In the Nurses’ Health research, the RCGP research and the Oxford Family Planning Association research, which are three large prospective cohort studies, neither long-lasting past COC use nor ongoing use was linked to an elevated risk of BC [5, 14, 17].
In a population-based, case–control study, which consisted of more than 4500 women with breast cancer, BC risk did not vary significantly between ongoing (relative risk (RR) 1.0, 95% CI: 0.8–1.3) or previous COC users (RR 0.9, 95% CI: 0.8–1.0) [15]. The RR did not arise persistently with long time use or with larger doses of oestrogen. For women with a family history of BC, the use of oral contraceptives was not associated with an elevated risk of breast cancer nor was the initiation of COC use earlier in life. Risk does not seem to be varied with different COC formulations [18].
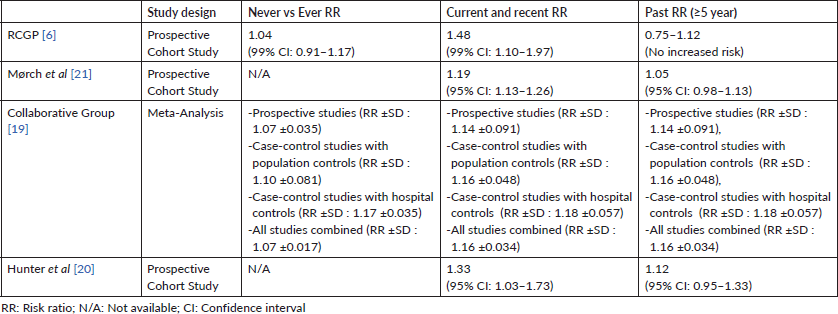
On the contrary, some articles have proposed a relationship between COC use and BC [6, 19–21] (Table 2). The results are diverse regarding the aforementioned categories of use. It is not known if this relationship is a biological effect or an outcome of increased diagnosis, whether different COC doses might have varying effects or a combination of reasons. However, the International Agency for Research on Cancer (IARC) Working Group on the Evaluation of Carcinogenic Risks to Humans released a monograph in 2007 and judged that there was adequate documentation to demonstrate the carcinogenicity of COCs in humans, with an elevated risk of BC in patients who were using them or had used them recently. Even so, if we inspect these articles that associate COC use with BC, the absolute risk is very low.
In a meta-analysis by the Collaborative Group on Hormonal Factors in Breast Cancer consisting of 54 studies in 25 countries and including over 53,000 women, COC use was related to an elevated risk of BC (RR 1.24, 95% CI: 1.15–1.33), which faded after cessation (RR 1.16 after 1–4 years, RR 1.07 after 5–9 years) and ceased after 10 or more years [19]. The length of COC use and the variety of COCs had no consequence on BC risk after adjusting for time of use and the malignancies detected then are less advanced clinically than the malignancies detected in never-users.
1.8 million women from a Danish registry were followed up for an average of 11 years in a prospective cohort study. In this research, ongoing or late COC users displayed an elevated risk of BC in contrast to counterparts who never gave preference to COCs (RR 1.19, 95% CI: 1.13–1.26) [21]. Derived risk for any hormonal contraception in comparison with non-users (RR 1.20, 95% CI: 1.14–1.26) was akin to this risk and rose with a longer period of usage. The overall rise in detected BC cases in the COC user group was small: about 1 added diagnose per 7,690 women annually. For women most likely to use COCs, who are below the age of 35 years, the overall enhanced risk was 1 added diagnosis per 50,000 women annually.
Findings from the Nurses’ Health Study, which is the largest cohort to date [22], including 121,577 women, reported that oral hormonal contraception usage is connected to elevated mortality due to BC for participants who have used OCs for 5 years or more in contrast with participants who never used COCs (RR, 95% CI: 1.26 (1.09–1.46)). In 2021, the Nurses’ Health Study II prospective cohort reported a higher risk for current users (HR 1.31; 95% CI: 1.09–1.58) compared to never-users. For former users, after 5 years of cessation, the risks were similar to those of never-users (23). However, the majority of studies do not show any association [17, 24–28].
In a recent meta-analysis, COC use and BC risk showed a significant linear dose–response relationship; 0.7% elevation in risk was reported with every 1 year increase of age [29].
Since there is no consensus in the literature on whether breast cancer is associated with COC use, it is wise to consider the details if the latter is true. Diagnosed breast cancer cases were less clinically advanced in ever-users compared to never-users [19]. Additionally, varying COC formulations may comprise varying risks of malignancy; continuing observations of these relationships may produce new information as COC formulations evolve. The RR of 1.2 (20% increase in risk) reported by Mørch [21] similar to that found by the Collaborative Group on Hormonal Factors in Breast Cancer [19] where the risk of breast cancer was 1.24 and comparable to preceding prospective studies [20, 30]. From this, it can be deduced that the malignancy risk for breast cancer with contemporary formulations is akin to the risk of older ones.
Combined oral contraceptives are not recommended for women with a personal history of BC (United States Medical Eligibility Criteria (MEC) category 4 (unacceptable risk) for current breast cancer, category 3 (risks outweigh the benefits) for past and no evidence of disease for 5 years) [31]. However, evidence [15, 19, 32–49] does not show an elevated risk for BC among women with either a family history of BC or BC susceptibility genes; therefore, women with breast cancer susceptibility genes (such as BRCA) or a family history of BC may use COCs at least for short term durations. Beside the point in a meta-analysis by Moorman et al [50], studying COC use among BRCA1 and BRCA2 mutation carriers, COC use had an elevated but non-statistically significant relationship with BC (odds ratio 1.21, 95% CI: 0.93–1.58).
Table 2. Studies reporting an increase in the risk of breast cancer associated with COC use.
Ovarian cancer
Ovarian cancer is the fifth leading cause of cancer death and the eight most common cancer in women [51]. Despite the progress in initial management, the rate of death for ovarian cancer continues to be uppermost among gynaecological cancers. Since ovarian malignancies characteristically appear at a more advanced stage clinically (with a parallel greater rate of death) than other frequent malignancies [51], there has been passionate concern in developing successful screening strategies. Unlike breast cancer, screening studies for ovarian cancer so far have not exhibited decreases in the rate of death and false-positive rates have been excessive [52–58].
COCs show a likely promising initial preventive measure for ovarian cancer. Researches have consistently demonstrated that continued use of COCs lowers the risk of ovarian cancer. Various broad pooled studies advocate that COCs provide a protective effect on ovarian malignancy risk, with a risk decrease of up to 50% with longer periods of COC use [59–62]. The largest pooled analysis today predicts that oral hormonal contraceptive pill use already has prevented 200,000 incidents of ovarian malignancy and 100,000 deaths from this cancer worldwide [60].
In a meta-analysis, which pooled 24 studies of oral hormonal contraception as primary protection for ovarian malignancy, ever-use was correlated with a decrease in ovarian malignancy in comparison with never-use (OR = 0.73, 95% CI: 0.66–0.81), with more than 50% decrease among participants with ≥10 years of usage [14]. Relationship between the degree of the protective effect and duration of COC use is correlated. The registry of this research consisted of both women who desired contraception and women who were pursuing a decrease in their possibility for ovarian malignancy. Separate meta-analyses have found comparable advantages in women at high risk for ovarian malignancy [15, 16].
In the aforementioned reanalysis by a collaborative group, risk reductions per 5 years of COC use were different for different histological subtypes; for serous, 22.1%; for endometrioid, 27.1%; for clear cell carcinomas, 21.3%; and lastly, 6.7% for mucinous tumours, which was non-significant [60]. Similar preventive ratios are obtained with tubal ligation; most reduction in endometrioid subtypes, less marked for serous neoplasms and for mucinous tumours, nil [63]. This is in concordance with hypothesises that suggest primary ovarian mucinous tumours are originated from germ cells [64] or Walthard cell nest [65], accordingly being less vulnerable to contraceptive or reproductive effects.
To understand the underlying mechanisms for how COCs prevent ovarian cancer, the process of ovarian cancer itself should be evaluated foremost. However, the aetiology of epithelial ovarian cancer has been debated for over more than half a century. It is known that ovarian cancer may develop through mutations with step-wise fashion (type 1, low-grade pathway) or much more aggressive mutations without precursor lesions causing widespread metastasis (type 2, high-grade pathway) [66]. Carcinogenesis theories assumed the coelomic epithelium of the ovary was the primary source for cells undergoing malignant degeneration [67–70]. It has been suggested that following every ovulation,
damage to the flat mesothelium was ending with entrapment of its cells during the healing process. Undergoing Müllerian metaplasia, they gave rise to mucinous, serous and endometrioid cystadenomas, which are cancer precursor lesions. But perplexities are born on the coelomic ancestry; most encountered mucinous, serous and endometrioid types are identical to intestinal/endocervical, fallopian tube and endometrium carcinomas, morphologically. Moreover, Müllerian cells are not present in ovaries. In addition to this, studies have failed to show a coelomic precursor lesion for these cancers, and therefore epithelial ovarian cancers are accepted as de novo [71].
Novel theories propose that the plantation of tubal and endometrial cells on ovary epithelium is the initial step for the process for these cancers of Müllerian origin [65, 72, 73] and retrograde menstruation provides the carcinogenic iron [74]. These theories are in concordance with the high prevalence of coexisting serous tubal intraepithelial carcinomas (STIC) in both BRCA1 and BRCA2 carriers undergoing risk-reducing salpingo-oophorectomy [75–80] and in women with non-hereditary high-grade serous ovarian cancers [81–83]. In addition to this, the relationship between clear cell/endometrioid cancers and endometriosis has been demonstrated [84–90]. By way of reduced tubal secretion and motility, ovulation inhibition, menstrual flow decreases and endometrial gland atrophy COCs may hamper the aforementioned pathways (Figure 2).
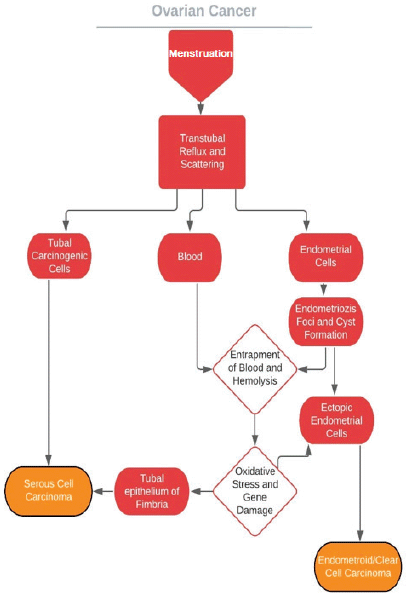
Figure 2. Probable mechanisms of ovarian cancers of Mullerian orgin.
BRCA1/2 carriers
There has been uneasiness that oral hormonal pregnancy prevention might elevate the risk of BC in susceptible populations like mutation carriers. Today, all women who are susceptible to gene mutation are proposed risk-reducing salpingo-oophorectomy (RR-BSO) at an age before which they are at the highest risk for ovarian malignancy as a routine practice; thus, COCs as protective measures are not required. Prophylactic surgeries are the standard of preventive care for these individuals. However, for women who must use COCs for their hormonal diseases or who have decided against RR-BSO, data advocate a decreased risk of ovarian malignancy in women with mutated susceptibility genes who take COC pills, although the hypothetical risk of elevated BC continues to remain. As discussed earlier, this risk was suggested by many studies but has not been consistently backed in the literature.
Women who possess the BRCA1 and BRCA2 genes compose a high-risk population and correspond to 10–15% of all BC cases. In a case–control study which consisted of nearly 2500 matched pairs of women who carried BRCA1 gene, oral hormonal contraception usage was related with an elevated risk of early onset BC, granted that medication commenced under the age of 20 (OR = 1.45, 95% CI: 1.20, 1.75) [91]; and the risk built up by 11% for each added year of use.
18 comparative, retrospective studies of oral hormonal contraceptive use in BRCA1/2 mutation carriers were analysed. This meta-analysis included 1503 cases of ovarian malignancy and 2855 cases of BC [92]. The results demonstrated that ever-use of oral contraceptives was related to a decreased risk of ovarian malignancy (RR 0.50, 95% CI: 0.33–0.75); this finding did not differ between mutation groups. A longer period of use raised preventive power. In 2021, this effect was also backed in a cohort [93]. The largest data in the analysis, a case–control study that included 798 women with ovarian malignancy, showed a 5% reduction in the risk of ovarian malignancy per year of COC use [94]. Moreover, COC use seems to be correlated with a reduced risk of fallopian tube malignancy in the common population [95].
In the study outlined above, there was no indication of a significantly elevated BC risk in COC users in general, for ongoing users or in the first decade after discontinuance of use [92].
In a meta-analysis deliberated beforehand, which studied COC use among BRCA1/2 mutation carriers, relationships between ever-use of oral contraceptives and ovarian and breast cancer among women who are carriers are akin to those shown for the common populace [50].
In a recent study, a prospective cohort study which comprised 6030 BRCA1 and 3809 BRCA2 carriers, retrospective and prospective analyses were inconsistent. In the prospective arm of BRCA1 carriers, there was no elevation in the risk of BC, but retrospective results were consistent between full-cohort (HR: 1.39) and left-truncated (HR: 1.26) analyses. The BRCA2 carriers’ prospective arm and full-cohort showed elevations in the risk of BC (HR: 1.75 and HR: 1.52, respectively) but retrospective results were inconsistent between full-cohort and left-truncated analyses (HR: 1.06) [96].
In a recent systematic review, overall data on BRCA mutation carriers were found to be very limited to decide the use of COCs in this population. BRCA1/2 mutation carriers also benefitted from the protective effects of COC use, which decreases the risk of ovarian cancer [97]. On the contrary, a new meta-analysis reported the elevated risk for breast cancer among long-term users (>5 years), while a protective effect for ovarian cancer was observed regardless of COC use [98]. Therefore, an elevation in BC risk cannot be ruled out. At any rate, cancer risk for ovaries is still raised up under COC use in this population and RR-BSO is favoured at a reasonable age [97].
Cervical cancer
GLOBOCAN predicted 570,000 cases and 311,000 deaths for cervical malignancy in 2018 and these numbers rank it to be the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death in women [99].
Human papillomavirus (HPV) is the essentially fundamental (but not enough) factor of cervical malignancy [100], which is classified as a group 1 carcinogen by the IARC working group with its 12 subtypes [101]. Immunosuppression (particularly HIV), tobacco use, parity and oral hormonal contraception use are other significant factors [102]. A far-reaching review overseen by IARC, as mentioned earlier, classified the use of COCs as carcinogenic to humans, and this was established on the stated relationships with cervical malignancy in part [103]. There is concern that HPV carriers may be at a notable risk for cervical malignancy [104]. Notwithstanding, screening for cervical malignancy should not be a precondition for the planning of pregnancy prevention strategies [105].
The age-specific incidence rate of cervical cancer increases after 25 years of age and peaks around 40 years in high-resource countries, although it continues to rise until 55–69 years of age in low-resource countries and so the contribution of COCs to the life-long incidence of cervical malignancy will revolve around on mostly the consequences later in life, when majority are former users. Long-term cervical malignancy risk does not seem to scale up among ever-users in comparison to never-users (incidence rate ratio 1.31, 99% CI 0.84–2.04) [6]. Yet, lamination by recency of COC use shows that ongoing or recent use (<5 years) is accompanied by elevated risk of cervical malignancy (incidence rate ratio 2.32, 99% CI 1.24–4.34) [6]. The reported risk seems to wane after relatively 5 years of ceasing COCs, with no indication of malignancy appearing at heightened risk in ever-users later in life.
In the EPIC study, which consisted of a cohort of over 300,000 participants who were followed up for 9 years, the relationship between hormonal cofactors and risk of acquiring cervical malignancy was assessed [106]. Similar to comparable studies, it has been found that cervical malignancy risk increased with continuation of use and waned after discontinuation, and the study cemented the acknowledged firm correlation between oral contraceptive use and cervical pre-cancer and cancer.
In a recent cohort study conducted in Denmark, nearly two million women of reproductive age were evaluated. Increased risk of cervical cancer was observed among recent and current users of COCs (RR 1.40 (95% CI: 1.28–1.53)). Risk analysis was similar for both adenocarcinoma and squamous cancer. This effect was observed more with the longer duration of use and declined after discontinuation. In this cohort, most women were unvaccinated against HPV; therefore, currently used COCs may have a similar risk profile compared to older formulations [107].
In a meta-analysis, which consisted of 24 studies comprising almost 17,000 patients with cervical malignancy and approximately 36,000 controls support this effect, the risk of cervical invasive malignancy was elevated among ongoing users of COCs with a prolonged period of use (RR ≥5 years usage versus never, 1.90 (95% CI: 1.69–2.13)). This enhanced risk diminished after discontinuation and was restored to those of never-users within 10 years. An identical model of risk was detected for both invasive and in-situ malignancy, and in high-risk HPV carriers [108].
The cervical cancer effects of COC usage is now known and elevated absolute risk in users is the valid measure of this consequence; however, it does not tell much about how exactly the HPV reliant carcinogenetic process is enhanced. COC use might, for instance, enhance the cervical vulnerability to HPV transmission and resultant infection or it might alter persistence or disposition of virus or the advancement or reversion of malign and pre-malign lesions. A plausible pathway to elucidate the relationships between oral contraceptive use and cervical cancer risk is that cervical tissue hormone receptors, especially progesterone, may be affected and the course of HPV may be altered. Particularly, hormones used in contraceptives are assumed to intensify the expression of HPV 16 E6 and E7 oncogenes, prompting the deterioration of p53 tumour suppressor genes and augmenting the capability of the viral DNA to mutate cells and promote neoplasticity [108–111] (Figure 3). Data from transgenic mouse models support the presumed pathway of oestrogen combined with HPV oncogenes inducing cervical carcinogenesis; however, these studies show that progesterone suppresses cervical carcinogenesis in mice [112, 113].
The latest 2015 WHO guidelines for MEC for contraceptive use categorises the recommendations into four groups. For HPV positivity, cervical intraepithelial neoplasies and cervical cancer awaiting treatment, the WHO recommendation group is 2; the advantages of usage outweigh the risks [114]. While using COCs among women with persistent human papillomavirus (HPV) infection and CINs, patients should be reminded that longer (>5 years) durations of use may increase the risk of carcinoma in situ and invasive carcinoma.
Endometrial cancer
The use of COCs provides prevention against endometrial neoplasia. Consistently, studies have found that the longer the period of usage, the more significant is the decrease in the risk of endometrial neoplasia. On average, every 5 years of use was related to a RR of 0.76; consequently, after approximately 10–15 years, the risk lowers by 50%. After discontinuation, the preventive influence goes on beyond 30 years, and does not give the impression to rely much on the oestrogen dose in formulations or individual traits like parity, body mass index or menopausal condition [115]. Since the rate of new cases of endometrial malignancy increases strongly later in life, the communal consequences of this converse relation lean primarily on the degree to which the decreased probability of endometrial malignancy carries on after discontinuation.
The decrease in cancer rate related to COC use appears to vary by malignancy form, being more effective for carcinomas (RR 0.69, 95% CI: 0.66–0.71) than sarcomas (0.83, 0.67–1.04; case-case comparison: p = 0·02)[115].
Findings from recent (2021) The Nurses’ Health Cohort Study II, including 107,069 women, showed oral hormonal contraception usage is connected to lower endometrial cancer risk (ever use, HR 0.77 [95% CI 0.65-0.91]; >10 years of use, 0.43 [0.32-0.58] vs. never OC use) [116]. These findings are consistent with previous meta-analysis and cohorts involving women who may have been exposed to higher dose formulations.
The use of COCs reduced the risk of endometrial cancer by 30%–40% in broad epidemiologic studies, and the risk decrease persevered for years after cessation [6, 115]. This advantage of hormonal contraception, which is assumably accurate for non-oral combined hormonal contraceptives as well, is thought to be mediated through progestogen ingredient which restrains endometrial proliferation and promotes differentiation. Progestin-only contraceptives seem to grant even stronger protection versus carcinogenesis of endometrium in epidemiologic studies [117–119]. On the other hand, in more former researches, COCs had larger doses compared to present-day formulations, which are progestogen prominent and hence may have preventive influences proportionate to progestogen-only preparations. This effect may be mediated through diminished exposure to unopposed oestrogen in the follicular phase, down regulation of oestrogen receptors and suppression of oestrogen mediated proliferation genes [120–122] (Figure 4). Moreover, it has also been shown that in women receiving perimenopausal HRT, the addition of progestins to the regime decreases the oestrogenic side effect on endometrial carcinogenesis [60, 123–125].
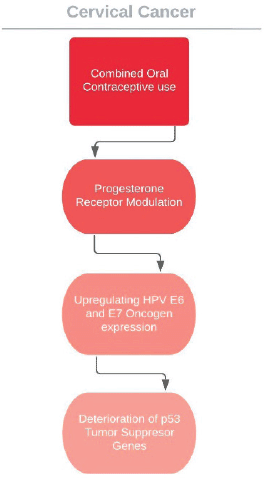
Figure 3. Suggested effect of COCs on HPV carcinogenesis.

Figure 4. Suggested effect of progestogens on endometrium.
Colorectal cancer
Colorectal cancer (CRC) is another common cancer in women, with relatively good survival rates that are at around 65%. The number of studies reporting on the relationship between COCs and CRC risk are voluminous and IARC has noted that the use of COCs may decrease the risk of colorectal cancer. For the past 20 years, still, there is no consensus between the results of epidemiological studies.
In a meta-analysis, which consisted of 23 studies comprising 14 case–control and 9 cohort studies, relative CRC risk for ever-use versus never-use was 0.8 [126]. In this study, the relationship between duration and risk was not evaluated but recent use was found to be more protective (OR = 0.7).
In another meta-analysis, which consisted of 29 studies comprising nearly 16,000 colorectal cancer cases, relative CRC risk for ever-use versus never-use was 0.8 [127]. In this study, the duration of COC use was inversely associated reduction of risk and effect was not dose-related. In the Royal College of General Practitioners’ Oral Contraception study, protection from CRC was presumed to be longer than 35 years [6].
In a recent population-based case–control study conducted in Northern Israel, nearly 3,000 CRC cases were evaluated. It was found that COC use was very inversely associated with CRC risk among people with Jewish and Arabic origin (ever-use versus never-use; odds ratio: 0.49 (0.39–0.62) and 0.14 (0.04–0.47), respectively) [128].
However, in some recent high-quality cohort studies, including the Nurses’ Health Study, neither long-lasting past COC use nor ongoing use was linked to an elevated risk of CRC [6, 104, 129–134] (Table 3). However, the studies were not consistent in their findings with risk reduction with prolonged use in comparison to the findings on ovarian and endometrial cancers [104, 132, 133].
In a cohort of 1.3 million women, which was followed up for 13 years, ever-use of COCs was found to be associated with an elevation in risk of anal cancer (OR = 1.51, 95% CI: 1.24–1.83) [135]. This may be associated with HPV-related pathways as in cervical cancer.
Several mechanisms, direct and indirect, were suggested for how COCs modify the risk for CRC [136] (Figure 5). Insulin-like growth factor regulation via oestrogen and body mass index is one of these suggestions [137]. Another would be reduction of secondary bile acids [138]: bile acids proliferative and carcinogenetic effects on colon cells were demonstrated in both rats and humans [139, 140]. Additionally, oestrogen has a direct growth-inhibiting effect on human colon cancer cell lines by way of its own receptor [141]. Oestrogen receptors are known to interact with different pathways detected in colon tumourigenesis [142, 143]. These tumour suppressive receptors are demonstrated to be diminished with age in colon tissue through methylation of the receptor gene and this gene may be upregulated by circulating oestrogen [144]. Even though more and more studies are being conducted on the mechanisms of cancer, such mechanisms are still waiting to be clarified.
COC use may decrease the CRC risk by at least 15% and this effect seems to not be dose-related, lasting for more than 30 years and not changing with duration of use.
Table 3. Studies analysing the risk of colorectal cancer associated with COC use.
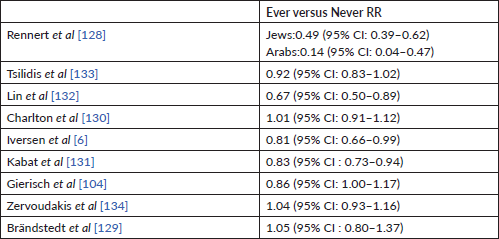

Figure 5. Proposed mechanisms for the effect of estrogen on colorectal cancer.
Liver cancer
Primary liver malignancy is the sixth most common diagnosed cancer and the ninth most common cancer in women, and it is mostly an issue in less advanced countries [99]. Hepatocellular carcinoma (HCC) is responsible for 70%–85% of primary live malignancy in majority of the regions [145].
The relationship between COC use and liver malignancy risk assessments has provided conflicting findings. In a meta-analysis of 12 case–control studies, which included over 700 women, the results relating to cancers, like HCC, are varied [146]. In one review, 6 of the studies showed between 2 and 20-fold increase in risk [146]; at the same time, a larger study revealed that COC use was not related to an increase in risk related to hepatic neoplasms [147].
Regarding the incidence of HCC, the effect of sex has been well demonstrated. Men are developing cancer more often than women, and this has brought about the hypothesis that female sex hormones may prevent hepatocellular carcinoma. Backing this, a case–control study, which consisted of 234 patients with treated HCC and 282 controls, demonstrated that post-menopausal HRT was decreasing the incidence of HCC (148). In a meta-analysis, it has been shown that people with differences in oestrogen receptor 1 gene had different odds of developing HCC (149). Furthermore, in a study which pooled nearly 800.000 women from 11 cohorts, surgical excision of both ovaries was significantly related to elevated odds of developing HCC (HR = 2.67; 95% CI: 1.22–5.85), after calculating for other individual and clinical cofactors and the length of HRT [150]. In the stated research, COC use was not related to an increase.
In a meta-analysis comprising 14 case–control studies and 3 cohort studies, a significant difference could not be detected; however, a detached investigation of these 14 case–control studies, contrary to the cohorts of the same meta-analysis, revealed a significant elevation in odds ratio (1.55) [147]. In a case-cohort investigation, which consisted of 267.400 female workers, 420 liver cancer cases were analysed with appropriate control group and odds ratio for COC use was not significant (95% CI: 0.82, 0.60–1.13) [7].
There is some evidence regarding the probable biochemical pathways through which hormones may promote carcinogenesis in liver. Hepatocytes carry oestrogen receptors, which are found to be upregulated in HCC, probably because of their proliferative and mutagenic effects [151, 152]. The increase in liver cancer remarked by IARC is not confirmed by the recent studies mentioned. Since IARC noted that the elevation of risk related to liver malignancy observed in regions is poor in cases of chronic liver disease and hepatitis B alone, it should be emphasised that these studies had populations in which hepatitis virus was prevalent.
The proliferation of cholangiocytes in the intrahepatic bile duct is upregulated by oestrogens. Cholangiocytes express both oestrogens receptor-α and -β and can develop intrahepatic cholangiocarcinoma (ICC), which is the most prevalent liver malignancy after HCC [153, 154]. Laboratory findings propose that oestrogens are cofactors for cholangiocarcinogenesis [155]; in the meantime, receptor modulators can suppress advancement [156–158]. In support of hormonal contribution to carcinogenesis, studies in the general population have revealed that higher oestrogen in circulation is related to elevated odds of ICC in both men and women [159, 160]. In a meta-analysis, which comprised 12 cohorts and over a million women, prolonged (over 9 years) COC use was related to a 62% elevation in ICC risk. Interestingly, in the study, hysterectomy was associated with nearly twofold risk of ICC but not oophorectomy; this may be due to prevalently seen cofactors in this group, such as adiposity, diabetes, HRT use or misclassification of the surgical procedures in cohorts [161].
Conclusive answers for myths
If COCs are carcinogenic, should we use other methods primarily?
Combined oral contraceptives are safe and effective and the effects are reversible. Patients who pursue family planning should be warned of possible carcinogenic outcomes, but it should also be explained that—in addition to sexual health advantages—preferring COCs may also decrease risks for endometrial, colorectal and ovarian cancers. To this day, in no study has cancer been shown as an enhanced mortality factor among COC users.
Can they be used by BRCA carriers and women with a family history of breast cancer?
The WHO MEC published in 2015 and US MEC for contraceptive use published in 2016 agree that the evidence to date does not propose an elevated risk for BC among women with either a family history of BC or BC susceptibility genes. Therefore, women with breast cancer susceptibility genes (such as BRCA) or a family history of BC may use COCs with safety [31, 114].
The European Society of Contraception and Reproductive Health Care published in 2018 has advocated against long-term use in BRCA carriers [162]. The UK MEC for contraceptive use (UK MEC) also take a comparable point of view placing COCs in category 3; theoretical or proven risks usually outweigh the advantages of using the method. However, it is clarified in the guideline that the very limited data in this area suggests the BC risk is not modified by COC use in both these high-risk groups [163].
BC risk elevation with COC use in this high-risk group is reported in some studies. On the other hand, others show no increase in risk. That being said, BRCA1/2 carriers should be notified that COC use may enhance BC risk. Early detection strategies for BC should be discussed with patients and periodic inspection should not be omitted; unfortunately, these strategies are not as effective for ovarian cancer and effective treatment is less likely to be achieved. The respective 5-year survival rates are 90% and 48% published by the American Cancer Society [164].
COCs can be used for contraceptive purposes in this population, but different forms of contraceptives should be elucidated. COC use for avoidance of ovarian cancer in cases where there is no need for birth control is still not advocated.
Are modern lower-dose formulations safer than ‘old’ formulations containing higher hormone dosages?
Since the development and approval of the first COC pill, formulations have evolved from comprising high-dose oestrogen (150 mcg) to very low doses (10 and 20 mcg). Oestrogens and progestogens are among the factors that may cause BC, and this has been confirmed, for instance, in HRT in post-menopausal population. Similar concerns were raised for reproductive women using COCs, but these claims were not given much attention because the evidence was on women using high-dose formulations of old. However, some recent high-quality studies on women using modern formulations found increased BC risk [21, 165]. This new information shows that risks with COCs that contain 20–50 μg ethinylestradiol may be comparable to those found in earlier studies. This cumulative dose of oestrogen may be the major risk factor for breast cancer [166].
Should we screen for HPV and cervical malignancy before prescribing COCs?
Screening for cervical malignancy should not be a precondition for the planning of pregnancy prevention strategies. The WHO MEC guideline acknowledges COCs as category 2 in women with HPV, CINs and cervical cancer, claiming that the advantages of COC use outweigh the risks in these groups. COCs enhance the risk with longer than 5 years of use and this enhanced risk diminishes after discontinuation and restores to those of never-users within 10 years.
Usually, cervical cancer progression is very slow, taking nearly a decade. Today, with comprehensive vaccination, widespread access to cervical cancer scanning and wider coverage of health services, elimination of cervical cancer is sought after. All women should be informed about sexually transmitted diseases, cervical cancer risk and screening strategies in polyclinic visits including family planning.
Conclusion
For a quarter century, combined oral contraceptive carcinogenicity has been held under a microscope. Recent studies reviewed support that, in addition to their effective contraceptive effect, COCs have a strong and long-lasting suppressive effect on endometrial, ovarian and colorectal cancers. Conversely, we see that some studies report the risk of cervical and breast cancer in recent use. Although the carcinogenic effects reported on breast and cervical cancer are reversible, the overall risk can further be reduced by methods such as lifestyle changes (e.g., lactation, smoking, exercise and weight control) or HPV vaccines. In addition, since cancers are more common in advancing ages, the cumulative protective effect of drugs used in the reproductive age may become more important than the reversible effect seen in the interim period.
In the same quarter century, medical practice shunned the paternalistic approach and shifted towards co-decision-making in counselling. It is important to discuss the benefit/harm balance with patients and make a decision specific to that patient. Once the patient population that should avoid combined oral contraceptives has been identified, it is not appropriate to make decisions on the remaining women solely for fear of cancer. Up-to-date and clear information flow will both strengthen the patient–doctor relationship and increase the patient’s compliance and confidence in the method chosen.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
1. Cogliano V, Grosse Y, and Baan R, et al (2005) Carcinogenicity of combined oestrogen-progestagen contraceptives and menopausal treatment Lancet Oncol 6(8) 552–553 https://doi.org/10.1016/S1470-2045(05)70273-4 PMID: 16094770
2. Grosse Y, Baan R, and Straif K, et al (2009) A review of human carcinogens--Part A: pharmaceuticals Lancet Oncol 10(1) 13 https://doi.org/10.1016/S1470-2045(08)70286-9 PMID: 19115512
3. Beral V, Hermon C, and Kay C, et al (1999) Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from Royal College of General Practitioners’ oral contraception study BMJ 318(7176) 96–100 https://doi.org/10.1136/bmj.318.7176.96 PMID: 9880284 PMCID: 27684
4. Colditz GA (1994) Oral contraceptive use and mortality during 12 years of follow-up: the Nurses’ Health Study Ann Intern Med 120(10) 821–826 https://doi.org/10.7326/0003-4819-120-10-199405150-00002 PMID: 8154642
5. Vessey M and Yeates D (2013) Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association contraceptive study Contraception 88(6) 678–683 https://doi.org/10.1016/j.contraception.2013.08.008 PMID: 24090961
6. Iversen L, Sivasubramaniam S, and Lee AJ, et al (2017) Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study Am J Obstet Gynecol 216(6) 580.e1–580.e9 https://doi.org/10.1016/j.ajog.2017.02.002
7. Rosenblatt KA, Gao DL, and Ray RM, et al (2009) Oral contraceptives and the risk of all cancers combined and site-specific cancers in Shanghai Cancer Causes Control 20(1) 27–34 https://doi.org/10.1007/s10552-008-9213-y PMCID: 2628413
8. Finlay-Schultz J and Sartorius CA (2015) Steroid hormones, steroid receptors, and breast cancer stem cells J Mammary Gland Biol Neoplasia 20(1–2) 39–50 https://doi.org/10.1007/s10911-015-9340-5 PMID: 26265122 PMCID: 4666507
9. Cavalieri E and Rogan E (2014) The molecular etiology and prevention of estrogen-initiated cancers: Ockham’s Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity Mol Aspects Med 36 1–55 https://doi.org/10.1016/j.mam.2013.08.002
10. Pasqualini JR (2007) Progestins and breast cancer Gynecol Endocrinol 23(sup1) 32–41 https://doi.org/10.1080/09513590701585003 PMID: 17943537
11. Kim JJ, Kurita T, and Bulun SE (2013) Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer Endocr Rev 34(1) 130–162 https://doi.org/10.1210/er.2012-1043 PMID: 23303565 PMCID: 3565104
12. Jordan SJ, Wilson LF, and Nagle CM, et al (2015) Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives Aust N Z J Public Health 39(5) 441–445 https://doi.org/10.1111/1753-6405.12444 PMID: 26437729 PMCID: 4606778
13. Sattin RW, Rubin GL, and Wingo PA, et al (1986) Oral-contraceptive use and the risk of breast cancer N Engl J Med 315(7) 405–411 https://doi.org/10.1056/NEJM198608143150701
14. Hankinson SE, Colditz GA, and Manson JE, et al (1997) A prospective study of oral contraceptive use and risk of breast cancer (Nurses’ Health Study, United States) Cancer Causes Control 8(1) 65–72 https://doi.org/10.1023/A:1018435205695 PMID: 9051324
15. Marchbanks PA, McDonald JA, and Wilson HG, et al (2002) Oral contraceptives and the risk of breast cancer N Engl J Med 346(26) 2025–2032 https://doi.org/10.1056/NEJMoa013202 PMID: 12087137
16. Michels KA, Pfeiffer RM, and Brinton LA, et al (2018) Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers JAMA Oncol 4(4) 516–521 https://doi.org/10.1001/jamaoncol.2017.4942 PMID: 29346467 PMCID: 5885214
17. Hannaford PC, Selvaraj S, and Elliott AM, et al (2007) Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner’s oral contraception study BMJ 335(7621) 651 https://doi.org/10.1136/bmj.39289.649410.55 PMID: 17855280 PMCID: 1995533
18. Marchbanks PA, Curtis KM, and Mandel MG, et al (2012) Oral contraceptive formulation and risk of breast cancer Contraception 85(4) 342–350 https://doi.org/10.1016/j.contraception.2011.08.007
19. Collaborative Group on Hormonal Factors in Breast Cancer (1996) Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies Lancet 347(9017) 1713–1727 PMID: 8656904
20. Hunter DJ, Colditz GA, and Hankinson SE, et al (2010) Oral contraceptive use and breast cancer: a prospective study of young women Cancer Epidemiol Biomarkers Prev 19(10) 2496–2502 https://doi.org/10.1158/1055-9965.EPI-10-0747 PMID: 20802021 PMCID: 3055790
21. Mørch LS, Skovlund CW, and Hannaford PC, et al (2017) Contemporary hormonal contraception and the risk of breast cancer N Engl J Med 377(23) 2228–2239 https://doi.org/10.1056/NEJMoa1700732 PMID: 29211679
22. Charlton BM, Rich-Edwards JW, and Colditz GA, et al (2014) Oral contraceptive use and mortality after 36 years of follow-up in the Nurses’ Health Study: prospective cohort study BMJ 349 g6356 https://doi.org/10.1136/bmj.g6356
23. Burchardt NA, Eliassen AH, and Shafrir AL, et al (2021) Oral contraceptive use by formulation and breast cancer risk by subtype in the Nurses’ Health Study II: a prospective cohort study Am J Obstet Gynecol S0002-9378(21)02686-7
24. Lu Y, Ma H, and Malone KE, et al (2011) Oral contraceptive use and survival in women with invasive breast cancer Cancer Epidemiol Biomark Prev 20(7) 1391–1397 https://doi.org/10.1158/1055-9965.EPI-11-0022
25. Nur U, El Reda D, and Hashim D, et al (2019) A prospective investigation of oral contraceptive use and breast cancer mortality: findings from the Swedish women’s lifestyle and health cohort BMC Cancer 19(1) 807 https://doi.org/10.1186/s12885-019-5985-6 PMID: 31412822 PMCID: 6694621
26. Trivers KF, Gammon MD, and Abrahamson PE, et al (2007) Oral contraceptives and survival in breast cancer patients aged 20 to 54 years Cancer Epidemiol Biomarkers Prev 16(9) 1822–1827 https://doi.org/10.1158/1055-9965.EPI-07-0053 PMID: 17855700
27. Vessey M, Yeates D, and Flynn S (2010) Factors affecting mortality in a large cohort study with special reference to oral contraceptive use Contraception 82(3) 221–229 https://doi.org/10.1016/j.contraception.2010.04.006 PMID: 20705149
28. Wingo PA, Austin H, and Marchbanks PA, et al (2007) Oral contraceptives and the risk of death from breast cancer Obstet Gynecol 110(4) 793–800 https://doi.org/10.1097/01.AOG.0000284446.22251.6e PMID: 17906011
29. Ji L-W, Jing C-X, and Zhuang S-L, et al (2019) Effect of age at first use of oral contraceptives on breast cancer risk: an updated meta-analysis Medicine 98(36) e15719 https://doi.org/10.1097/MD.0000000000015719 PMID: 31490359 PMCID: 6738995
30. Romieu I, Willett WC, and Colditz GA, et al (1989) Prospective study of oral contraceptive use and risk of breast cancer in women J Natl Cancer Inst 81(17) 1313–1321 https://doi.org/10.1093/jnci/81.17.1313 PMID: 2769784
31. Curtis KM, Tepper NK, and Jatlaoui TC, et al (2016) U.S. Medical Eligibility Criteria for Contraceptive Use, 2016 MMWR Recomm Rep 65(3) 1–103 PMID: 27467319
32. Collaborative Group on Hormonal Factors in Breast Cancer Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease Lancet 358(9291) 1389–1399 PMID: 11705483
33. Black MM, Barclay TH, and Polednak A, et al (1983) Family history, oral contraceptive usage, and breast cancer Cancer 51(11) 2147–2151 https://doi.org/10.1002/1097-0142(19830601)51:11<2147::AID-CNCR2820511133>3.0.CO;2-X PMID: 6839302
34. Brinton LA, Hoover R, and Szklo M, et al (1982) Oral contraceptives and breast cancer Int J Epidemiol 11(4) 316–322 https://doi.org/10.1093/ije/11.4.316 PMID: 7152784
35. Brohet RM, Goldgar DE, and Easton DF, et al (2007) Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: a report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group J Clin Oncol 25(25) 3831–3836 https://doi.org/10.1200/JCO.2007.11.1179 PMID: 17635951
36. Claus EB, Stowe M, and Carter D (2003) Oral contraceptives and the risk of ductal breast carcinoma in situ Breast Cancer Res Treat 81(2) 129–136 https://doi.org/10.1023/A:1025728524310 PMID: 14572155
37. Grabrick DM, Hartmann LC, and Cerhan JR, et al (2000) Risk of breast cancer with oral contraceptive use in women with a family history of breast cancer JAMA 284(14) 1791–1798 https://doi.org/10.1001/jama.284.14.1791 PMID: 11025831
38. Gronwald J, Byrski T, and Huzarski T, et al (2006) Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland Breast Cancer Res Treat 95(2) 105–109 https://doi.org/10.1007/s10549-005-9051-5
39. Haile RW, Thomas DC, and McGuire V, et al (2006) BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50 Cancer Epidemiol Biomarkers Prev 15(10) 1863–1870 https://doi.org/10.1158/1055-9965.EPI-06-0258 PMID: 17021353
40. Harris NV, Weiss NS, and Francis AM, et al (1982) Breast cancer in relation to patterns of oral contraceptive use Am J Epidemiol 116(4) 643–651 https://doi.org/10.1093/oxfordjournals.aje.a113447 PMID: 7137151
41. Hennekens CH, Speizer FE, and Lipnick RJ, et al (1984) A case-control study of oral contraceptive use and breast cancer J Natl Cancer Inst 72(1) 39–42 https://doi.org/10.1093/jnci/72.1.39 PMID: 6363789
42. Jernström H, Loman N, and Johannsson OT, et al (2005) Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing Eur J Cancer 41(15) 2312–2320 https://doi.org/10.1016/j.ejca.2005.03.035 PMID: 16118051
43. Milne RL, Knight JA, and John EM, et al (2005) Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations Cancer Epidemiol Biomarkers Prev 14(2) 350–356 https://doi.org/10.1158/1055-9965.EPI-04-0376 PMID: 15734957
44. Narod SA, Dubé MP, and Klijn J, et al (2002) Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers J Natl Cancer Inst 94(23) 1773–1779 https://doi.org/10.1093/jnci/94.23.1773 PMID: 12464649
45. Rosenberg L, Palmer JR, and Rao RS, et al (1996) Case-control study of oral contraceptive use and risk of breast cancer Am J Epidemiol 143(1) 25–37 https://doi.org/10.1093/oxfordjournals.aje.a008654 PMID: 8533744
46. Silvera SA, Miller AB, and Rohan TE (2005) Oral contraceptive use and risk of breast cancer among women with a family history of breast cancer: a prospective cohort study Cancer Causes Control 16(9) 1059–1063 https://doi.org/10.1007/s10552-005-0343-1 PMID: 16184471
47. Ursin G, Henderson BE, and Haile RW, et al (1997) Does oral contraceptive use increase the risk of breast cancer in women with BRCA1/BRCA2 mutations more than in other women? Cancer Res 57(17) 3678–3681 PMID: 9288771
48. Ursin G, Ross RK, and Sullivan-Halley J, et al (1998) Use of oral contraceptives and risk of breast cancer in young women Breast Cancer Res Treat 50(2) 175–184 https://doi.org/10.1023/A:1006037823178 PMID: 9822222
49. Gaffield ME, Culwell KR, and Ravi A (2009) Oral contraceptives and family history of breast cancer Contraception 80(4) 372–380 https://doi.org/10.1016/j.contraception.2009.04.010 PMID: 19751860
50. Moorman PG, Havrilesky LJ, and Gierisch JM, et al (2013) Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis J Clin Oncol 31(33) 4188–4198 https://doi.org/10.1200/JCO.2013.48.9021 PMID: 24145348
51. Altekruse SF, Kosary CL, and Krapcho M, et al (2010) SEER Cancer Statistics Review, 1975–2007 (Bethesda: National Cancer Institute)
52. Buys SS, Partridge E, and Black A, et al (2011) Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial JAMA 305(22) 2295–2303 https://doi.org/10.1001/jama.2011.766 PMID: 21642681
53. Buys SS, Partridge E, and Greene MH, et al (2005) Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial Am J Obstet Gynecol 193(5) 1630–1639 https://doi.org/10.1016/j.ajog.2005.05.005 PMID: 16260202
54. Jacobs I, Skates SJ, and Macdonald N (2000) Ovarian cancer screening was feasible but did not decrease incidence of index cancer or mortality Western J Med 172 97 https://doi.org/10.1136/ewjm.172.2.97
55. Jacobs IJ, Skates SJ, and MacDonald N, et al (1999) Screening for ovarian cancer: a pilot randomised controlled trial Lancet 353(9160) 1207–1210 https://doi.org/10.1016/S0140-6736(98)10261-1 PMID: 10217079
56. Menon U, Gentry-Maharaj A, and Hallett R, et al (2009) Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol 10(4) 327–340 https://doi.org/10.1016/S1470-2045(09)70026-9 PMID: 19282241
57. van Nagell JR, Jr., DePriest PD, and Reedy MB, et al (2000) The efficacy of transvaginal sonographic screening in asymptomatic women at risk for ovarian cancer Gynecol Oncol 77(3) 350–356 https://doi.org/10.1006/gyno.2000.5816 PMID: 10831341
58. van Nagell JR, Jr., DePriest PD, and Ueland FR, et al (2007) Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened Cancer 109(9) 1887–1896 https://doi.org/10.1002/cncr.22594 PMID: 17373668
59. Beral V, Bull D, and Green J, et al (2007) Ovarian cancer and hormone replacement therapy in the Million Women Study Lancet 369(9574) 1703–1710 https://doi.org/10.1016/S0140-6736(07)60534-0 PMID: 17512855
60. Beral V, Doll R, and Hermon C, et al (2008) Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls Lancet 371(9609) 303–314. https://doi.org/10.1016/S0140-6736(08)60167-1 PMID: 18294997
61. Bosetti C, Negri E, and Trichopoulos D, et al (2002) Long-term effects of oral contraceptives on ovarian cancer risk Int J Cancer 102(3) 262–265 https://doi.org/10.1002/ijc.10696 PMID: 12397647
62. Negri E, Franceschi S, and Tzonou A, et al (1991) Pooled analysis of 3 European case-control studies: I. Reproductive factors and risk of epithelial ovarian cancer Int J Cancer 49(1) 50–56 https://doi.org/10.1002/ijc.2910490110 PMID: 1874569
63. Cibula D, Widschwendter M, and Májek O, et al (2011) Tubal ligation and the risk of ovarian cancer: review and meta-analysis Hum Reprod Update 17(1) 55–67 https://doi.org/10.1093/humupd/dmq030
64. Scully RE (1979) Tumors of the ovary and maldeveloped gonads Atlas Tumor Pathol 2(16) 152–173
65. Kurman RJ and Shih Ie-M (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory Am J Surg Pathol 34(3) 433–443 https://doi.org/10.1097/PAS.0b013e3181cf3d79 PMID: 20154587 PMCID: 2841791
66. Ricciardelli C and Oehler MK (2009) Diverse molecular pathways in ovarian cancer and their clinical significance Maturitas 62(3) 270–275 https://doi.org/10.1016/j.maturitas.2009.01.001 PMID: 19193504
67. Fathalla MF (1971) Incessant ovulation--a factor in ovarian neoplasia? Lancet 2(7716) 163 https://doi.org/10.1016/S0140-6736(71)92335-X PMID: 4104488
68. Cramer DW and Welch WR (1983) Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis J Natl Cancer Inst 71(4) 717–721 PMID: 6578367
69. Resta L, Russo S, and Colucci GA, et al (1993) Morphologic precursors of ovarian epithelial tumors Obstet Gynecol 82(2) 181–186 PMID: 8336861
70. Auersperg N, Wong AS, and Choi KC, et al (2001) Ovarian surface epithelium: biology, endocrinology, and pathology Endocr Rev 22(2) 255–288 PMID: 11294827
71. Bell DA and Scully RE (1994) Early de novo ovarian carcinoma. A study of fourteen cases Cancer 73(7) 1859–1864 https://doi.org/10.1002/1097-0142(19940401)73:7<1859::AID-CNCR2820730714>3.0.CO;2-L PMID: 8137211
72. Levanon K, Crum C, and Drapkin R (2008) New insights into the pathogenesis of serous ovarian cancer and its clinical impact J Clin Oncol 26(32) 5284–5293 https://doi.org/10.1200/JCO.2008.18.1107 PMID: 18854563 PMCID: 2652087
73. Salvador S, Gilks B, and Köbel M, et al (2009) The fallopian tube: primary site of most pelvic high-grade serous carcinomas Int J Gynecol Cancer 19(1) 58–64 https://doi.org/10.1111/IGC.0b013e318199009c PMID: 19258943
74. Huang X (2003) Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal Mutat Res 533(1–2) 153–171 https://doi.org/10.1016/j.mrfmmm.2003.08.023 PMID: 14643418
75. Piek JM, van Diest PJ, and Zweemer RP, et al (2001) Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer J Pathol 195(4) 451–456 https://doi.org/10.1002/path.1000 PMID: 11745677
76. Leeper K, Garcia R, and Swisher E, et al (2002) Pathologic findings in prophylactic oophorectomy specimens in high-risk women Gynecol Oncol 87(1) 52–56 https://doi.org/10.1006/gyno.2002.6779 PMID: 12468342
77. Carcangiu ML, Radice P, and Manoukian S, et al (2004) Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers Int J Gynecol Pathol 23(1) 35–40 https://doi.org/10.1097/01.pgp.0000101082.35393.84
78. Olivier RI, van Beurden M, and Lubsen MA, et al (2004) Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up Br J Cancer 90(8) 1492–1497 https://doi.org/10.1038/sj.bjc.6601692 PMID: 15083174 PMCID: 2409718
79. Finch A, Shaw P, and Rosen B, et al (2006) Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers Gynecol Oncol 100(1) 58–64 https://doi.org/10.1016/j.ygyno.2005.06.065
80. Medeiros F, Muto MG, and Lee Y, et al (2006) The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome Am J Surg Pathol 30(2) 230–236 https://doi.org/10.1097/01.pas.0000180854.28831.77 PMID: 16434898
81. Kindelberger DW, Lee Y, and Miron A, et al (2007) Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship Am J Surg Pathol 31(2) 161–169 https://doi.org/10.1097/01.pas.0000213335.40358.47 PMID: 17255760
82. Carlson J, Roh MH, and Chang MC, et al (2008) Recent advances in the understanding of the pathogenesis of serous carcinoma: the concept of low- and high-grade disease and the role of the fallopian tube Diagn Histopathol 14(8) 352–365 https://doi.org/10.1016/j.mpdhp.2008.06.009
83. Roh MH, Kindelberger D, and Crum CP (2009) Serous tubal intraepithelial carcinoma and the dominant ovarian mass: clues to serous tumor origin? Am J Surg Pathol 33(3) 376–383 https://doi.org/10.1097/PAS.0b013e3181868904
84. Vercellini P, Parazzini F, and Bolis G, et al (1993) Endometriosis and ovarian cancer Am J Obstet Gynecol 169(1) 181–182 https://doi.org/10.1016/0002-9378(93)90159-G PMID: 8392791
85. Seidman JD (1996) Prognostic importance of hyperplasia and atypia in endometriosis Int J Gynecol Pathol 15(1) 1–9 https://doi.org/10.1097/00004347-199601000-00001 PMID: 8852439
86. Somigliana E, Vigano P, and Parazzini F, et al (2006) Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence Gynecol Oncol 101(2) 331–341 https://doi.org/10.1016/j.ygyno.2005.11.033 PMID: 16473398
87. Viganó P, Somigliana E, and Chiodo I, et al (2006) Molecular mechanisms and biological plausibility underlying the malignant transformation of endometriosis: a critical analysis Hum Reprod Update 12(1) 77–89 https://doi.org/10.1093/humupd/dmi037
88. Clement PB (2007) The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects Adv Anat Pathol 14(4) 241–260 https://doi.org/10.1097/PAP.0b013e3180ca7d7b PMID: 17592255
89. Viganò P, Somigliana E, and Parazzini F, et al (2007) Bias versus causality: interpreting recent evidence of association between endometriosis and ovarian cancer Fertil Steril 88(3) 588–593 https://doi.org/10.1016/j.fertnstert.2006.11.180 PMID: 17320873
90. Kobayashi H (2009) Ovarian cancer in endometriosis: epidemiology, natural history, and clinical diagnosis Int J Clin Oncol 14(5) 378–382 https://doi.org/10.1007/s10147-009-0931-2 PMID: 19856043
91. Kotsopoulos J, Lubinski J, and Moller P, et al (2014) Timing of oral contraceptive use and the risk of breast cancer in BRCA1 mutation carriers Breast Cancer Res Treat 143(3) 579–586 https://doi.org/10.1007/s10549-013-2823-4 PMID: 24458845
92. Iodice S, Barile M, and Rotmensz N, et al (2010) Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis Eur J Cancer 46(12) 2275–2284 https://doi.org/10.1016/j.ejca.2010.04.018 PMID: 20537530
93. Schrijver LH, Antoniou AC, and Olsson H, et al (2021) Oral contraceptive use and ovarian cancer risk for BRCA1/2 mutation carriers: an international cohort study Am J Obstet Gynecol 225(1) 51.e1–51.e17 https://doi.org/10.1016/j.ajog.2021.01.014
94. McLaughlin JR, Risch HA, and Lubinski J, et al (2007) Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study Lancet Oncol 8(1) 26–34 https://doi.org/10.1016/S1470-2045(06)70983-4 PMID: 17196508
95. Jordan SJ, Green AC, and Whiteman DC, et al (2008) Serous ovarian, fallopian tube and primary peritoneal cancers: a comparative epidemiological analysis Int J Cancer 122(7) 1598–1603 https://doi.org/10.1002/ijc.23287
96. Schrijver LH, Olsson H, and Phillips KA, et al (2018) Oral contraceptive use and breast cancer risk: retrospective and prospective analyses from a BRCA1 and BRCA2 mutation carrier cohort study JNCI Cancer Spectr 2(2) pky023 https://doi.org/10.1093/jncics/pky023
97. Huber D, Seitz S, and Kast K, et al (2020) Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: a systematic review Arch Gynecol Obstet 301(4) 875–884 https://doi.org/10.1007/s00404-020-05458-w PMID: 32140806 PMCID: 8494665
98. Park J, Huang D, and Chang YJ, et al (2022) Oral contraceptives and risk of breast cancer and ovarian cancer in women with a BRCA1 or BRCA2 mutation: a meta-analysis of observational studies Carcinogenesis 43(3) 231–242 https://doi.org/10.1093/carcin/bgab107
99. Bray F, Ferlay J, and Soerjomataram I, et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68(6) 394–424 https://doi.org/10.3322/caac.21492 PMID: 30207593
100. Walboomers JM, Jacobs MV, and Manos MM, et al (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide J Pathol 189(1) 12–19 https://doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F PMID: 10451482
101. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2007) Human papillomaviruses IARC Monogr Eval Carcinog Risks Hum 90 1–636
102. Thun M, Linet MS, and Cerhan JR, et al (2017) Cancer Epidemiology and Prevention (Oxford: Oxford University Press)
103. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Combined estrogen-progestogen contraceptives and combined estrogen-progestogen menopausal therapy IARC Monogr Eval Carcinog Risks Hum 91 1–528 PMID: 18756632 PMCID: 4781221
104. Gierisch JM, Coeytaux RR, and Urrutia RP, et al (2013) Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review Cancer Epidemiol Biomarkers Prev 22(11) 1931–1943 https://doi.org/10.1158/1055-9965.EPI-13-0298 PMID: 24014598
105. Stewart FH, Harper CC, and Ellertson CE, et al (2001) Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence JAMA 285(17) 2232–2239 https://doi.org/10.1001/jama.285.17.2232 PMID: 11325325
106. Roura E, Travier N, and Waterboer T, et al (2016) The Influence of Hormonal Factors on the Risk of Developing Cervical Cancer and Pre-Cancer: Results from the EPIC Cohort PLoS One 11(1) e0147029 https://doi.org/10.1371/journal.pone.0147029 PMID: 26808155 PMCID: 4726518
107. Iversen L, Fielding S, and Lidegaard Ø, et al (2021) Contemporary hormonal contraception and cervical cancer in women of reproductive age Int J Cancer https://doi.org/10.1002/ijc.33585 PMID: 33818778
108. Appleby P, Beral V, and Berrington de González A, et al (2007) Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies Lancet 370(9599) 1609–1621 https://doi.org/10.1016/S0140-6736(07)61684-5 PMID: 17993361
109. Gadducci A, Barsotti C, and Cosio S, et al (2011) Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: a review of the literature Gynecol Endocrinol 27(8) 597–604 https://doi.org/10.3109/09513590.2011.558953 PMID: 21438669
110. Moodley M, Moodley J, and Chetty R, et al (2003) The role of steroid contraceptive hormones in the pathogenesis of invasive cervical cancer: a review Int J Gynecol Cancer 13(2) 103–110 https://doi.org/10.1136/ijgc-00009577-200303000-00001 PMID: 12657108
111. Rinaldi S, Plummer M, and Biessy C, et al (2011) Endogenous sex steroids and risk of cervical carcinoma: results from the EPIC study Cancer Epidemiol Biomarkers Prev 20(12) 2532–2540 https://doi.org/10.1158/1055-9965.EPI-11-0753 PMID: 21994406
112. Chung SH, Franceschi S, and Lambert PF (2010) Estrogen and ERalpha: culprits in cervical cancer? Trends Endocrinol Metab 21(8) 504–511 https://doi.org/10.1016/j.tem.2010.03.005 PMID: 20456973 PMCID: 2914219
113. Yoo YA, Son J, and Mehta FF, et al (2013) Progesterone signaling inhibits cervical carcinogenesis in mice Am J Pathol 183(5) 1679–1687 https://doi.org/10.1016/j.ajpath.2013.07.026 PMID: 24012679 PMCID: 3816255
114. World Health Organization (2015) Medical Eligibility Criteria for Contraceptive Use (Geneva: WHO)
115. Collaborative Group on Epidemiological Studies on Endometrial Cancer (2015) Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies Lancet Oncol 16(9) 1061–1070 https://doi.org/10.1016/S1470-2045(15)00212-0 PMID: 26254030
116. Burchardt NA, Shafrir AL, and Kaaks R, et al (2021) Oral contraceptive use by formulation and endometrial cancer risk among women born in 1947–1964: the Nurses’ Health Study II, a prospective cohort study Eur J Epidemiol 36(8) 827–839 https://doi.org/10.1007/s10654-020-00705-5 PMCID: 8416825
117. Cullins VE (1996) Noncontraceptive benefits and therapeutic uses of depot medroxyprogesterone acetate J Reprod Med 41(5 Suppl) 428–433 PMID: 8725706
118. Kaunitz AM (1996) Depot medroxyprogesterone acetate contraception and the risk of breast and gynecologic cancer J Reprod Med 41(5 Suppl) 419–427 PMID: 8725705
119. Soini T, Hurskainen R, and Grénman S, et al (2014) Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland Obstet Gynecol 124(2 Pt 1) 292–299 https://doi.org/10.1097/AOG.0000000000000356 PMID: 25004338
120. Key TJ and Pike MC (1988) The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk Br J Cancer 57(2) 205–212 https://doi.org/10.1038/bjc.1988.44 PMID: 3358913 PMCID: 2246441
121. Clarke CL and Sutherland RL (1990) Progestin regulation of cellular proliferation Endocr Rev 11(2) 266–301 https://doi.org/10.1210/edrv-11-2-266 PMID: 2114281
122. Maxwell GL, Schildkraut JM, and Calingaert B, et al (2006) Progestin and estrogen potency of combination oral contraceptives and endometrial cancer risk Gynecol Oncol 103(2) 535–540 https://doi.org/10.1016/j.ygyno.2006.03.046 PMID: 16740300
123. Weiderpass E, Adami HO, and Baron JA, et al (1999) Risk of endometrial cancer following estrogen replacement with and without progestins J Natl Cancer Inst 91(13) 1131–1137 https://doi.org/10.1093/jnci/91.13.1131 PMID: 10393721
124. Doherty JA, Cushing-Haugen KL, and Saltzman BS, et al (2007) Long-term use of postmenopausal estrogen and progestin hormone therapies and the risk of endometrial cancer Am J Obstet Gynecol 197(2) 139.e1–139.e7 https://doi.org/10.1016/j.ajog.2007.01.019
125. Allen NE, Tsilidis KK, and Key TJ, et al (2010) Menopausal hormone therapy and risk of endometrial carcinoma among postmenopausal women in the European Prospective Investigation Into Cancer and Nutrition Am J Epidemiol 172(12) 1394–1403 https://doi.org/10.1093/aje/kwq300 PMID: 20961969
126. Bosetti C, Bravi F, and Negri E, et al (2009) Oral contraceptives and colorectal cancer risk: a systematic review and meta-analysis Hum Reprod Update 15(5) 489–498 https://doi.org/10.1093/humupd/dmp017 PMID: 19414526
127. Luan NN, Wu L, and Gong TT, et al (2015) Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies Cancer Causes Control 26(1) 65–78 https://doi.org/10.1007/s10552-014-0483-2
128. Rennert G, Rennert HS, and Pinchev M, et al (2020) Hormonal and reproductive factors and reduction in the risk of colorectal cancer Eur J Cancer Prev 29(3) 229–237 https://doi.org/10.1097/CEJ.0000000000000538
129. Brändstedt J, Wangefjord S, and Nodin B, et al (2014) Associations of hormone replacement therapy and oral contraceptives with risk of colorectal cancer defined by clinicopathological factors, beta-catenin alterations, expression of cyclin D1, p53, and microsatellite-instability BMC Cancer 14 371 https://doi.org/10.1186/1471-2407-14-371 PMID: 24885829 PMCID: 4041054
130. Charlton BM, Wu K, and Zhang X, et al (2015) Oral contraceptive use and colorectal cancer in the Nurses’ Health Study I and II Cancer Epidemiol Biomarkers Prev 24(8) 1214–1221 https://doi.org/10.1158/1055-9965.EPI-15-0172 PMID: 26063479 PMCID: 4526380
131. Kabat GC, Miller AB, and Rohan TE (2008) Oral contraceptive use, hormone replacement therapy, reproductive history and risk of colorectal cancer in women Int J Cancer 122(3) 643–646 https://doi.org/10.1002/ijc.23079
132. Lin J, Zhang SM, and Cook NR, et al (2007) Oral contraceptives, reproductive factors, and risk of colorectal cancer among women in a prospective cohort study Am J Epidemiol 165(7) 794–801 https://doi.org/10.1093/aje/kwk068 PMID: 17215381
133. Tsilidis KK, Allen NE, and Key TJ, et al (2010) Oral contraceptives, reproductive history and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition Br J Cancer 103(11) 1755–1759 https://doi.org/10.1038/sj.bjc.6605965 PMID: 21045829 PMCID: 2994229
134. Zervoudakis A, Strickler HD, and Park Y, et al (2011) Reproductive history and risk of colorectal cancer in postmenopausal women J Natl Cancer Inst 103(10) 826–834 https://doi.org/10.1093/jnci/djr101 PMID: 21447807 PMCID: 3096797
135. Coffey K, Beral V, and Green J, et al (2015) Lifestyle and reproductive risk factors associated with anal cancer in women aged over 50 years Br J Cancer 112(9) 1568–1574 https://doi.org/10.1038/bjc.2015.89 PMID: 25867258 PMCID: 4453684
136. Newcomb PA, Pocobelli G, and Chia V (2008) Why hormones protect against large bowel cancer: old ideas, new evidence Adv Exp Med Biol 617 259–269 https://doi.org/10.1007/978-0-387-69080-3_24 PMID: 18497049
137. Slattery ML, Ballard-Barbash R, and Edwards S, et al (2003) Body mass index and colon cancer: an evaluation of the modifying effects of estrogen (United States) Cancer Causes Control 14(1) 75–84 https://doi.org/10.1023/A:1022545017867 PMID: 12708728
138. McMichael AJ and Potter JD (1980) Reproduction, endogenous and exogenous sex hormones, and colon cancer: a review and hypothesis J Natl Cancer Inst 65(6) 1201–1207 PMID: 7001123
139. Reddy BS, Watanabe K, and Weisburger JH, et al (1977) Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats Cancer Res 37(9) 3238–3242 PMID: 884672
140. McKeown-Eyssen G (1994) Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev 3(8) 687–695 PMID: 7881343
141. Lointier P, Wildrick DM, and Boman BM (1992) The effects of steroid hormones on a human colon cancer cell line in vitro Anticancer Res 12(4) 1327–1330 PMID: 1503430
142. Smirnoff P, Liel Y, and Gnainsky J, et al (1999) The protective effect of estrogen against chemically induced murine colon carcinogenesis is associated with decreased CpG island methylation and increased mRNA and protein expression of the colonic vitamin D receptor Oncol Res 11(6) 255–264
143. Schwartz B, Smirnoff P, and Shany S, et al (2000) Estrogen controls expression and bioresponse of 1,25-dihydroxyvitamin D receptors in the rat colon Mol Cell Biochem 203(1–2) 87–93 https://doi.org/10.1023/A:1007015027268 PMID: 10724336
144. Issa JP, Ottaviano YL, and Celano P, et al (1994) Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon Nat Genet 7(4) 536–540 https://doi.org/10.1038/ng0894-536 PMID: 7951326
145. Venook AP, Papandreou C, and Furuse J, et al (2010) The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective Oncologist 15(S4) 5–13 https://doi.org/10.1634/theoncologist.2010-S4-05 PMID: 21115576
146. Maheshwari S, Sarraj A, and Kramer J, et al (2007) Oral contraception and the risk of hepatocellular carcinoma J Hepatol 47(4) 506–513 https://doi.org/10.1016/j.jhep.2007.03.015 PMID: 17462781
147. An N (2015) Oral contraceptives use and liver cancer risk: a dose-response meta-analysis of observational studies Medicine 94(43) e1619 https://doi.org/10.1097/MD.0000000000001619 PMID: 26512555 PMCID: 4985369
148. Hassan MM, Botrus G, and Abdel-Wahab R, et al (2017) Estrogen replacement reduces risk and increases survival times of women with hepatocellular carcinoma Clin Gastroenterol Hepatol 15(11) 1791–1799 https://doi.org/10.1016/j.cgh.2017.05.036 PMID: 28579181 PMCID: 5901750
149. Sun X, Duan J, and Zou Y, et al (2015) Impact of multipath effects on theoretical accuracy of TOA-based indoor VLC positioning system Photonics Res 3(6) 296–299 https://doi.org/10.1364/PRJ.3.000296
150. McGlynn KA, Sahasrabuddhe VV, and Campbell PT, et al (2015) Reproductive factors, exogenous hormone use and risk of hepatocellular carcinoma among US women: results from the Liver Cancer Pooling Project Br J Cancer 112(7) 1266–1272 https://doi.org/10.1038/bjc.2015.58 PMID: 25742475 PMCID: 4385955
151. Lindberg MC (1992) Hepatobiliary complications of oral contraceptives J Gen Intern Med 7(2) 199–209 https://doi.org/10.1007/BF02598014 PMID: 1336797
152. De Benedetti VM, Welsh JA, and Yu MC, et al (1996) p53 mutations in hepatocellular carcinoma related to oral contraceptive use Carcinogenesis 17(1) 145–149 https://doi.org/10.1093/carcin/17.1.145 PMID: 8565124
153. Alvaro D, Barbaro B, and Franchitto A, et al (2006) Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma Am J Pathol 169(3) 877–888 https://doi.org/10.2353/ajpath.2006.050464 PMID: 16936263 PMCID: 1698823
154. Alvaro D, Alpini G, and Onori P, et al (2002) Alfa and beta estrogen receptors and the biliary tree Mol Cell Endocrinol 193(1–2) 105–108 https://doi.org/10.1016/S0303-7207(02)00103-X PMID: 12161009
155. Thun M, Linet MS, and Cerhan JR, et al (2017) Cancer Epidemiology and Prevention 4th edn (New York: Oxford University Press) p 1328
156. Sampson LK, Vickers SM, and Ying W, et al (1997) Tamoxifen-mediated growth inhibition of human cholangiocarcinoma Cancer Res 57(9) 1743–1749 PMID: 9135018
157. Mancino A, Mancino MG, and Glaser SS, et al (2009) Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor Dig Liver Dis 41(2) 156–163 https://doi.org/10.1016/j.dld.2008.02.015 PMCID: 2626155
158. Isse K, Specht SM, and Lunz JG, 3rd, et al (2010) Estrogen stimulates female biliary epithelial cell interleukin-6 expression in mice and humans Hepatology 51(3) 869–880 https://doi.org/10.1002/hep.23386 PMID: 20043322
159. Petrick JL, Florio AA, and Zhang X, et al (2020) Associations Between prediagnostic concentrations of circulating sex steroid hormones and liver cancer among postmenopausal women Hepatology 72(2) 535–547 https://doi.org/10.1002/hep.31057
160. Hunsawong T, Singsuksawat E, and In-chon N, et al (2012) Estrogen is increased in male cholangiocarcinoma patients’ serum and stimulates invasion in cholangiocarcinoma cell lines in vitro J Cancer Res Clin Oncol 138(8) 1311–1320 https://doi.org/10.1007/s00432-012-1207-1 PMID: 22476540
161. Petrick JL, McMenamin ÚC, and Zhang X, et al (2020) Exogenous hormone use, reproductive factors and risk of intrahepatic cholangiocarcinoma among women: results from cohort studies in the Liver Cancer Pooling Project and the UK Biobank Br J Cancer 123(2) 316–324 https://doi.org/10.1038/s41416-020-0835-5 PMID: 32376888 PMCID: 7374167
162. Health ESoCaR (2018) Medical condition: hormonal contraception and breast and ovarian cancer [https://escrheu/wp-content/uploads/2018/10/update_mc_chc_breast_ovarian_cancer_me13-06-notespdf] Date accessed: 10/01/21]
163. Percy L (2016) The new UK Medical Eligibility Criteria (UKMEC): what has changed? J Fam Planning Reprod Health Care 42(2) 81–82 https://doi.org/10.1136/jfprhc-2016-101488
164. Society AC 5-year relative survival, 2009–2015, by cancer type [https:// cancerstatisticscentercancerorg/#!] Date accessed: 10/01/21
165. Busund M, Bugge NS, and Braaten T, et al (2018) Progestin‐only and combined oral contraceptives and receptor‐defined premenopausal breast cancer risk: the Norwegian Women and Cancer Study Int J Cancer 142(11) 2293–2302 https://doi.org/10.1002/ijc.31266 PMID: 29349773 PMCID: 5893363
166. Lund E, Dumeaux V, and Braaten T, et al (2007) Cohort Profile: The Norwegian Women and Cancer Study—NOWAC—Kvinner og kreft Int J Epidemiol 37(1) 36–41 https://doi.org/10.1093/ije/dym137 PMID: 17644530





