Mortality prediction in women with corpus uteri cancer in Brazil: a 21-year analysis
Diego Bessa Dantas1, Lucio Flávio Garcia Rodrigues1, Fabiana de Campos Gomes2 and João Simão de Melo-Neto1
1Federal University of Pará (UFPA), Belém, PA, Brazil
2School of Medicine of São José do Rio Preto (FAMERP), São José do Rio Preto, SP, Brazil
Abstract
Mortality data obtained from the Mortality Information System identified a total of 19,499 deaths in women caused by corpus uteri cancer in Brazil. However, the association between mortality and sociodemographic factors in these women is not fully understood. A study based on the secondary data on deaths caused by corpus uteri cancer recorded in the SIM-DATASUS was conducted. Deaths reported from 1996 to 2016 in the health information system were included. Sociodemographic factors were analysed to determine their association with mortality. Low schooling is highly associated with mortality in all administrative regions. Advanced age, race and marital status have specific association with mortality for the different geographic regions. Black, Brown and Indigenous women with low schooling and of advanced age are highly associated with mortality. Brown, White and Black women of advanced age had the highest corpus uteri cancer related mortality rates. Women with low schooling who died of corpus uteri cancer were either single or widows. The marital status of Black, White and Brown women aged <59 years was single. The sociodemographic factors that predict mortality in women with corpus uteri cancer in Brazil were presented and can be used to guide public health.
Keywords: Brazil, uterine neoplasms, mortality, epidemiology
Correspondence to: João Simão de Melo-Neto
Email: jsmeloneto@ufpa.br
Published: 04/05/2020
Received: 28/08/2019
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Tumours of the corpus uteri are divided into the following two main groups: endometrial tumours and mesenchymal tumours. The former are common gynaecological diseases, whereas the latter manifest more aggressively and are rarer with worse prognosis than the former. Endometrioid adenocarcinomas and carcinosarcomas or leiomyosarcomas, considering clear types of tumours alone, are the most common types of endometrial tumours and mesenchymal tumours, respectively [1].
The proportion of adenocarcinomas accounts for greater than 80% of all corpus uteri cancers in all countries studied, except in Brazil (74.1%). In Brazil, the proportion of unspecified morphology is comparatively high (13.1%), and the proportion of sarcoma is low and is approximately 1.2%–5.1% of all corpus uteri cancers [2].
Approximately, 95% of uterine malignancies are endometrial carcinoma [3]. Worldwide, the incidence of endometrial cancer is rapidly increasing, with the highest disease burden reported in North America and Western Europe [4]. However, the epidemiological data associated with mortality in Brazil are unknown.
The onset of corpus utreri cancer is usually in postmenopausal women. Its occurrence and mortality are highly associated with overweight and obese women [5]. Additionally, understanding the association between the sociodemographic factors (geographic region, age, marital status, race and schooling) and mortality caused by corpus uteri cancers aids in the development of public policies aimed at the most vulnerable population.
Mortality data obtained from the Mortality Information System (SIM) of the Brazilian Ministry of Health, available on the DATASUS website with annual data collected from 1996 to 2016, identified a total of 19,499 deaths of women caused by corpus uteri cancer in Brazil [6]. Comparative studies have shown an association between mortality prediction, survival and sociodemographic factors in women with corpus uteri cancer, supporting the need to increase the number of studies that present consistent data on the subjects [7].
This study aimed to analyse the sociodemographic factors that predict mortality in corpus uteri cancer in Brazil. Specifically, the sociodemographic factors (geographic regions, age, race/ethnicity and schooling) will be evaluated to determine their association with mortality from 1996 to 2016.
Methods
Ethics
This study analyses secondary data available in the DATASUS. The data are publicised with unrestricted use and access. Ethical assessment of the research ethics committee is not required according to the terms of the National Health Council Resolution No. 466 of December 12, 2012.
Type of study
An analytic, descriptive and retrospective study based on secondary data on deaths caused by corpus uteri cancer recorded in the SIM of the Ministry of Health of Brazil was conducted.
Database
The SIM is a secondary database available in the Informatics Department of the Brazilian National Health System (DATASUS) of the Ministry of Health [8]. Deaths reported from 1996 to 2016 in Brazil in the health information system, and classified by the International Classification of Diseases [9], defined according to the 10th revision by code C54 (43), were included.
Study variables
Geographic regions, age, marital status, race/ethnicity and educational attainment were considered the sociodemographic factors. These factors were further categorised as follows: geographic regions (North, Northeast, Midwest, South and Southeast), race/ethnicity (Brown, White, Black, Yellow and Indigenous), age (less than 19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79 and greater than 80 years), marital status (single, married, widowed and divorced) and schooling (no schooling greater than 12 years).
Statistical analysis
The data were submitted for descriptive and inferential analysis. For the description of data, absolute and relative frequencies were used. Age-period-cohort (APC) analysis using a suitable model that accounts for the identification problem to discern variations in mortality due to independent effects of age groups, calendar time periods of death and birth cohorts was performed. For all analysed variables in this study, the following functions have been estimated: net drift (overall annual percentage change in accordance with calendar period and birth cohort); local drifts (annual percentage changes for each age group in accordance with calendar period and birth cohort); all age deviations (fitted longitudinal and cross-sectional age curves are log-linear); all period deviations (fitted temporal trends and period rate ratios are log-linear); all cohorts deviations (cohort rate ratios are log-linear and all local drifts equal the net drift); and all period (or cohort) rate ratios (RR) (age incidence pattern in every period (or cohort)). Wald test was used to verify difference significative, being considered p < 0.05. We obtained these estimable parameters by the APC Web Tool (Biostatistics Branch, National Cancer Institute, Bethesda, MD, USA) [10]. The chi-squared test with Yates’s correction as used to analyse the association between sociodemographic factors and mortality caused by corpus uteri cancer. To quantify the level of association, odds ratios with 95% confidence intervals (95% CI) for the occurrences of death in women with corpus uteri cancer were used.
Results
The highest number of deaths from uterine cancer was observed in women with the following characteristics: aged 60 to 79 years (59.02%), belonging to the White race (61.44%), with low education ≤3 (31.58%), married (34.84%) or widowed (33.54%) and reside in the Midwest (56.33%) of Brazil. The results are shown in Figure 1.
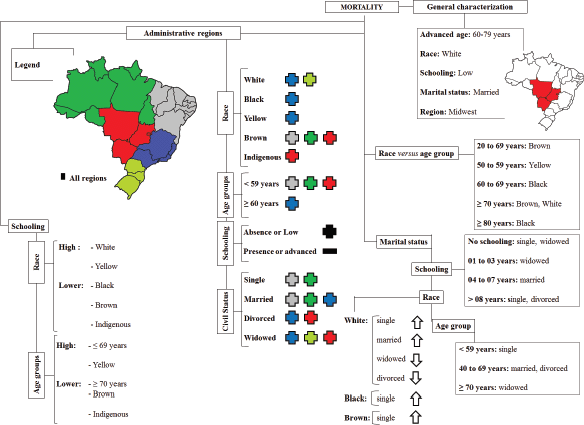
Figure 1. Diagram showing the sociodemographic factors associated with mortality caused by corpus uteri cancer.
APC analysis
The results obtained in the analysis of the APC are seen in Figure 2. During the period of 1996 to 2016, the net drift, that represented annual percentage change of the expected age-adjusted rates, was 3.237% (95% CI: 1.539–4.964) per year. Local drift values and cohorts’ deviations are not statistically significant. All age deviations demonstrated that there is greater risk of progressing to death with advancing age in relation to the younger individuals progressively until the last years of life, with a greater peak after 80 years of age (Figure 2B). On the other hand, younger women had a lower risk with RR <1 up to 38 years of age. All period deviations demonstrated that fitted temporal trends and period RR (Figure 2C) are log-linear, indicating that age pattern of patients that death in every period with increase in recent years. All cohort RR indicated an age incidence pattern in every birth cohort (Figure 2D).
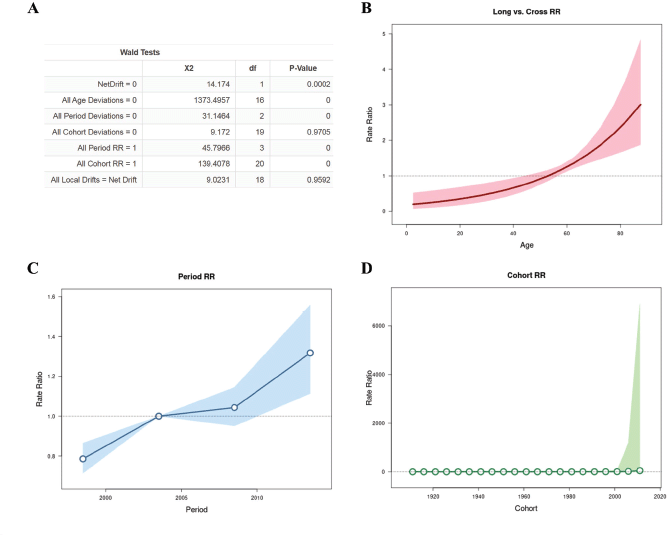
Figure 2. APC analysis with Wald test (A), all age deviations (B), period rate ratios (RR) (C) and cohort (RR) (D) .
Administrative regions versus race, age group, schooling and marital status
The association between administrative regions and race, age group, schooling and marital status is presented in Table 1.
Table 1. Association between geographic regions and race, age group, schooling and marital status.
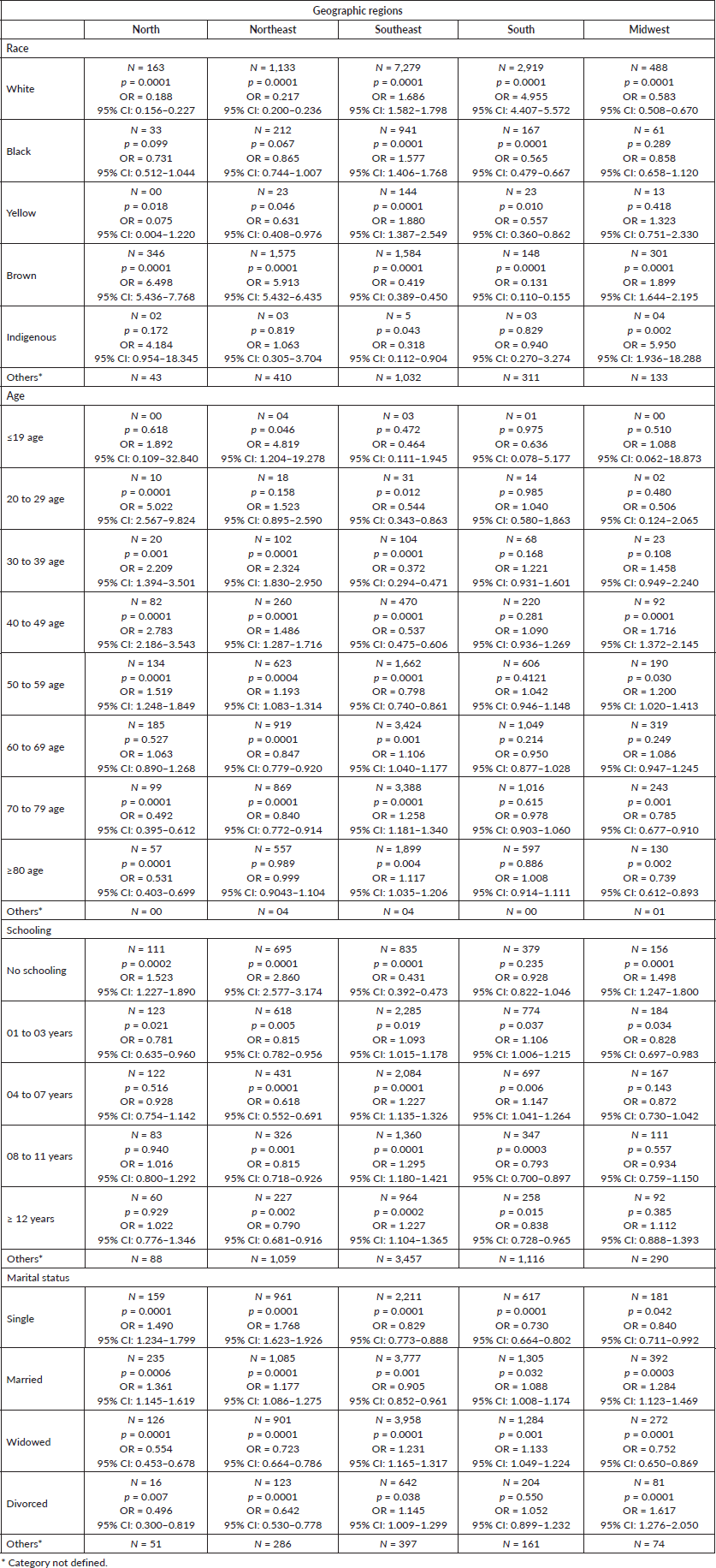
According to the sociodemographic factors, the association between geographic region and race and mortality was as follows: White women from the South and Southeast regions, Black and Yellow women from the Southeast region, and Brown women from the North, Northeast, and Midwest regions had six times higher chance of mortality than those from the rest of the regions. Indigenous women from the Midwest region were highly associated with mortality, with five times higher chance of mortality compared to those from the rest of the regions.
Hence, an association between women’s’ geographic region and age and mortality caused by corpus uteri cancer was observed, and from these data it was, women aged <19 years in the Northeast region; women aged 50–59 years in the North, Northern and Midwest regions; and women aged 60–69, 70–79 and greater than 80 years in the Southeast region were highly associated with mortality.
Regarding the level of schooling, women from the North, Northeast, and Midwest regions with no schooling, women from the South and Southeast regions with 1–7 years of experience in schooling, and women from the Southeast region with 8–11 years and ≥12 years of experience in schooling were highly associated with mortality caused by corpus uteri cancer. However, women who presently study (North, Northeast and Midwest regions) or had advanced schooling (South region) exhibited lower odds of mortality. Interestingly, there were lower odds of mortality in the Southeast region.
According to the data on the marital status of women, single and married women from the North and the Northeast regions, married and widowed women from the South region, widowed and divorced women from the Southeast region and married and divorced women from the Midwest region were highly associated with mortality caused by corpus uteri cancer.
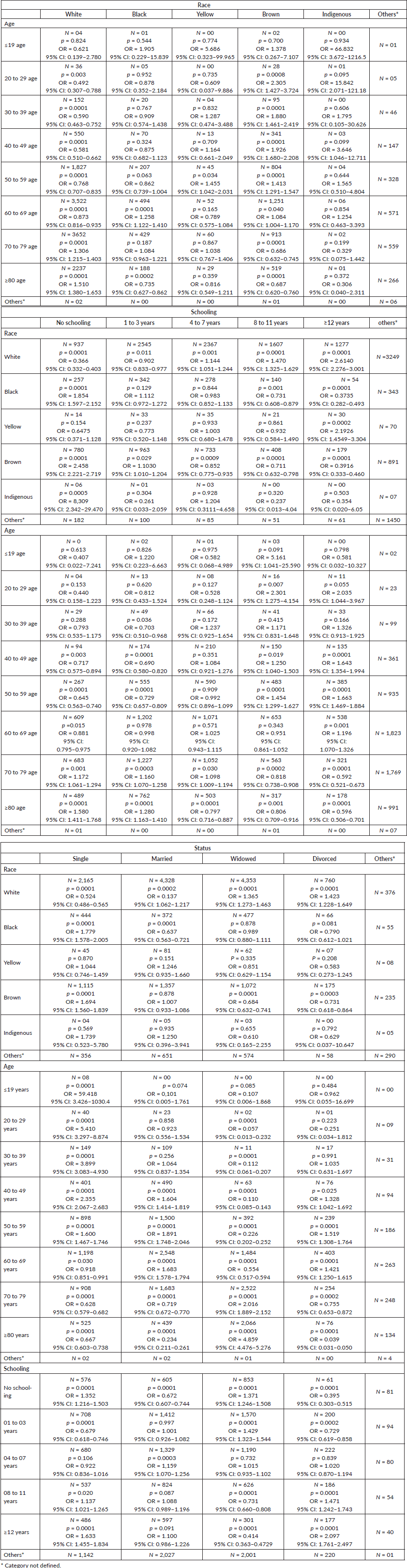
Race versus age group
The association between race and age group and mortality caused by corpus uteri cancer is presented in Table 2. White women older than 70 years, Black women aged 60–69 years, Yellow women aged 50–59 years and Brown women aged 20–69 years were highly associated with mortality.
Schooling versus race and age group
White and Yellow women with high education and Black, Brown and Indigenous women with low education were highly associated with mortality. White women with low education and Black with high educational level had lower odds of mortality. Indigenous women with high education and Yellow women with low education had no association with mortality. Women aged ≤69 years with high level of education and women aged ≥70 years with low level of education were highly associated with mortality.
Marital status versus race, age group and schooling
The association between marital status, race, age group and schooling and mortality caused by corpus uteri cancer is presented in Table 2. Single and widowed women with no schooling and widowed women who had 1–3 years of experience in schooling, married women with 4–7 years of experience in schooling, and women with ‘8–11 years’ and ‘greater than 12 years’ of experience in schooling were highly associated with mortality.
The association between age group and mortality caused by corpus uteri cancer was as follows: single women aged <59 years were highly associated with mortality, and women aged <19 years had 59 times higher chance of mortality. Married and divorced women aged 40–69 years were highly associated with mortality. Moreover, widowed women aged ≥70 years were associated with mortality, with women aged greater than 80 years having five times higher chance of mortality.
Additionally, White and single and married women exhibited lower odds of mortality, while widowed and divorced women were positively associated with mortality. Moreover, Black and Brown single women were highly associated with mortality.
Table 2. Association between marital status and race, age group and schooling.
Discussion
Corpus uteri cancer is a very common gynaecological malignancy, especially in high-income countries. Although the overall prognosis is relatively good, high-grade corpus uteri cancer tends to recur. Recurrence needs to be prevented since the prognosis for cancer recurrence is worse than the initial cancer. This study analysed the sociodemographic factors that predict mortality caused by corpus uteri cancer in Brazil [11]. Specifically, the sociodemographic factors (geographic regions, age, race and schooling) were evaluated to determine their association with mortality from 1996 to 2016.
The results of APC analysis revealed that mortality is higher with increasing age. Black women aged 60–69 years, Yellow women aged 50–59 years, and Brown women aged 20–69 years were highly associated with mortality, with results showing that higher chance of mortality was noted even in younger women. The association between elderly women and mortality caused by corpus uteri cancer is well understood in the literature, showing a higher risk mortality in elderly women in relation to younger women [12].
According to a study using the data from Brazil, Black women presented a higher percentage of corpus uteri cancer progression or recurrence compared to non-Black women, and all of these women benefited from the public health services offered, a common characteristic that makes this group homogeneous [13].
Cancer health differences are often described as the unequal burden of cancer deaths in one racial/ethnic group compared to another. For example, the National Cancer Statistics in the USA shows that death from 9 out of the top 10 cancers in men and women is mostly observed in Blacks. Considering that there is no association between genetic and biological variances for these differences, it is possible to associate these results with the unequal distribution of the social determinants of health as the primary cause of cancer differences [14].
It was confirmed that Black, Brown and Indigenous women with low schooling have a greater association with mortality and White and Yellow women with a high level of schooling. It can be hypothesised that the low schooling group has greater difficulty in accessing healthcare services compared to the other groups. Low educational levels can lead to low health literacy; hence, women with high educational level are able to access, understand, and act on complex health information and communicate with healthcare personnel [14]. However, in relation to the group with a high level of schooling, according to epidemiological studies, it is possible that their greater purchasing power is highly associated with obesity [15]. Hence, the association between obesity and cancer has to be considered [16].
According to the presented results on marital status, there is a lower association between mortality and married women compared to other marital statuses, supporting other studies [17, 18] that associate single women, including widows, with significantly higher risk of metastatic cancer, resulting even in death, than married women. The importance of this study is that it highlights the consistent and substantial impact that marriage status has on cancer. The general hypothesis between these studies was that married women have a greater social support system than single women, which improves their overall health maintenance, including medication adherence [19].
It was observed that in the North and Northeast regions, mortality was higher in Brown women aged less than 60 years, with emphasis on the association in Indigenous women living in the Midwest region. These women have difficulty accessing the health policies in the country, mainly due to geographical and cultural barriers [20].
These results are possibly associated with women in these regions having higher difficulty accessing the oncological treatment centres compared to the South and Southeast regions, where the highest number of mortality is observed in women aged greater than 60 years [21]. The structural differences between the different regions in Brazil in the public health system lead women to migrate to search for better conditions in treating their diseases. In addition to the discomfort experienced by women, care is focused on large healthcare centres, causing an overload on the current healthcare capacity [22].
The results of the analysis support the initial hypothesis that the interval between cancer diagnosis and early treatment is longer for women with vulnerable social characteristics, regardless of the stage of the disease, compared to women with no vulnerable social characteristics. There is a clear consensus in the literature that the shorter the interval between diagnosis and treatment, the better the prognosis and patient survival. Immediate action is essential to the effectiveness of treatment in more advanced stages of the disease or patient comfort in palliative treatment [23].
Understanding the association between sociodemographic factors and mortality caused by corpus uteri cancer is essential for the development of public policies worldwide, but in Brazil, similar to other developing countries, it is necessary to recognise that there are limitations on the quality of data collection. A very high number of unknown or unreported data that greatly undermine the reliability of the analysis performed in studies using secondary banks are noted. On the contrary, the number of unknown data has declined over the years. Another limitation is characterised by the change of terms and items in the collection worksheets, reducing the standardisation in the collection and data releases in the platform [24].
Conclusion
In this study, we found that the sociodemographic factors of race, age, schooling, marital status and geographic regions present specific characteristics that predict mortality in women with corpus uteri cancer in Brazil. These findings can be used to review or develop new public health guidelines and policies. Thus, there is a need to improve the existing public policies to prevent death caused by corpus uteri cancer, especially for the most vulnerable population with less social support and greater difficulty in accessing oncological healthcare services.
Conflict of interest
The authors have no conflicts of interest to disclose.
Authors’ contributions
The authors participated in all the stages of the study.
Funding statement
No funding was received for this work.
References
1. Brany D, Dvorska D, Nachajova M, et al. Malignant tumors of the uterine corpus: molecular background of their origin Tumor Biol 36(9) 6615–6621 https://doi.org/10.1007/s13277-015-3824-1
2. Hori M and Katanoda K (2015) Morphological distribution of cervical and corpus uteri cancer from Cancer Incidence in Five Continents Vol. X Jpn J Clin Oncol 45(7) 697 https://doi.org/10.1093/jjco/hyv097 PMID: 26130689
3. Bhurgri Y, Nazir K, Shaheen Y, et al (2007) Patho-epidemiology of cancer corpus uteri in Karachi South’1995–1997’ Asian Pac J Cancer Prev 8(4) 489–494
4. Rahib L, Smith BD, Aizenberg R, et al (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States Cancer Res 74(11) 2913–2921 https://doi.org/10.1158/0008-5472.CAN-14-0155 PMID: 24840647
5. Song Y-M, Sung J, and Ha M (2008) Obesity and risk of cancer in postmenopausal Korean women J Clin Oncol 26(20) 3395–3402 https://doi.org/10.1200/JCO.2007.15.7867 PMID: 18612154
6. Ministério da Saúde (2019) Secretaria Executiva. DATASUS. Informações de Saúde [http://www2.datasus.gov.br/DATASUS/index.php?area=02] Date accessed: 06/05/19
7. Galvin A, Delva F, Helmer C, et al. Sociodemographic, socioeconomic, and clinical determinants of survival in patients with cancer: a systematic review of the literature focused on the elderly J Geriatr Oncol 9(1) 6–14 https://doi.org/10.1016/j.jgo.2017.07.007 PMID: 29030150
8. Alves CM, Guerra MR, and Bastos RR (2009) Cervical cancer mortality trends in Minas Gerais State, Brazil, 1980–2005 Cadernos de saude publica 25(8) 1693–1700 https://doi.org/10.1590/S0102-311X2009000800005 PMID: 19649410
9. World Health Organization (2004) International Classification of Diseases 10th Revision (Geneva, Switzerland: World Health Organization)
10. Rosenberg PS, Check DP, and Anderson WF (2014) A web tool for Age-period-cohort analysis of cancer incidence and mortality rates Cancer Epidemiol Biomark Prev 23 2296–2302 https://doi.org/10.1158/1055-9965.EPI-14-0300
11. Amant F, Mirza MR, and Carien L (2012) Creutzberg. Cancer of the corpus uteri Int J Gynecol Obst 119(S2) https://doi.org/10.1016/S0020-7292(12)60024-1
12. Nolen SC, Evans MA, Fischer A, et al (2017) Cancer—incidence, prevalence and mortality in the oldest-old. A comprehensive review Mech Ageing Dev 164 113–126 https://doi.org/10.1016/j.mad.2017.05.002 PMID: 28502820
13. Márquez-Magaña L, Samayoa C, and Umanzor C (2013) Debunking ‘race’and asserting social determinants as primary causes of cancer health disparities: outcomes of a science education activity for teens J Cancer Educ 28(2) 314–318 https://doi.org/10.1007/s13187-013-0474-0 PMID: 23532632
14. Asare M, Flannery M, and Kamen C (2017) Social determinants of health: a framework for studying cancer health disparities and minority participation in research Oncol Nurs Forum 44(1) 20–23 NIH Public Access, 2017 https://doi.org/10.1188/17.ONF.20-23 PMID: 28060469 PMCID: 5583708
15. Ferreira RA and Benicio MH (2015) Obesity in Brazilian women: association with parity and socioeconomic status Rev Panamericana de salud publica 37 (4–5) 337–342
16. Ackerman SE, Blackburn OA, and Marchildon F, et al Insights into the link between obesity and cancer Curr Obes Rep 6(2) 195–203 PMID: 28434109
17. Aizer AA, McCarthy EP, and Mendu ML, et al (2013) Marital status and survival in patients with cancer J Clin Oncol 31(31) 3869 https://doi.org/10.1200/JCO.2013.49.6489 PMID: 24062405 PMCID: 4878087
18. Kim A, Ashman P, Ward-Peterson M, et al (2017) Racial disparities in cancer-related survival in patients with squamous cell carcinoma of the esophagus in the US between 1973 and 2013 PLoS One 12(8) e0183782 https://doi.org/10.1371/journal.pone.0183782 PMID: 28832659 PMCID: 5568373
19. Chaves, Mde B, Cardoso AM, and Almeida C (2006) Implementation of indigenous people’s health policy in Angra dos Reis, Rio de Janeiro, Brazil: obstacles and prospects Cad Saude Publica 22(2) 295–305
20. Braga SFM, de Souza MC, and Cherchiglia ML (2017) Time trends for prostate cancer mortality in Brazil and its geographic regions: an age–period–cohort analysis Cancer Epidemiol 50 53–59 https://doi.org/10.1016/j.canep.2017.07.016 PMID: 28810175
21. Cubero DIG, Sette CVM, and Piscopo BCP, et al (2018) Epidemiological profile of Brazilian oncological patients seen by a reference oncology center of the public health system and who migrate in search of adequate health care Rev Assoc Méd Bras 64(9) 814–818 https://doi.org/10.1590/1806-9282.64.09.814
22. Cabral ALLV, Giatti L, and Casale C, et al (2019) Social vulnerability and breast cancer: differentials in the interval between diagnosis and treatment of women with different sociodemographic profiles Cien Saude Colet 24(2) 613–622 https://doi.org/10.1590/1413-81232018242.31672016 PMID: 30726393
23. Souza MC, Vasconcelos AG, and Cruz OG (2012) Trends in lung cancer mortality in Brazil from the 1980s into the early 21st century: age-period-cohort analysis Cad Saude Publica 28 21–30 https://doi.org/10.1590/S0102-311X2012000100003 PMID: 22267062
24. Carstensen B (2007) Age–period–cohort models for the Lexis diagram Stat Med 26(15) 3018–3045 https://doi.org/10.1002/sim.2764

