
By Dr Bhawna Sirohi and Shaheenah Dawood
The American Society of Clinical Oncology (ASCO) 2018 Annual Meeting was exciting for breast cancer this year with a plenary presentation and numerous potential practice changing and hypothesis generating studies presented.
We saw the results of numerous trials trying to refine standard chemotherapeutic regimens and trying to de-escalate treatment regimens to make them kinder for our patients.
Here, we review some of the abstracts that we found interesting at ASCO 2018.
1. De-escalating treatments
Chemotherapy
In the NHS, all patients with node negative, ER positive early breast cancer (EBC) with an intermediate risk of recurrence (Nottingham prognostic index score of between 3.4 and 5.4) are entitled to have their tumour sent for oncotype-DX assay testing to determine benefit from adjuvant chemotherapy.
With a recurrence score (RS) of >= 31 (considered high risk), adjuvant chemotherapy clearly reduces the risk of recurrence of disease.
A RS between 18 and 30 was traditionally considered to be associated with intermediate risk of recurrence of disease with the clinical utility associated with chemotherapy being of indeterminate benefit.
An individualised discussion between the clinician and patient drove shared decision making with little evidence to support or not support chemotherapy.
The TailorX study1 (trial assigning individualised options for treatment) randomised patients with intermediate RS to endocrine therapy (ET) versus ET and chemotherapy (CT).
Unlike, real world practice, the cut-off points for intermediate RS was re-calibrated to 11-25 and not 15-30.
6,711 patients with early stage node negative breast cancer whose tumours exhibited a RS between 11 and 25 were randomised.
63% had a tumour size of 1-2 cm, 57% had grade 2 disease, 33% were younger than 50 years of age and the most common chemotherapy regimen used as adjuvant CT was docetaxel-cyclophospmaide (56%).
36% of patients received an anthracycline containing regimen.
The authors reported that the primary end point of invasive disease free survival (iDFS) was not significantly different between the two randomised arms and the secondary end points of relapse free interval and overall survival (OS) were also similar.
An exploratory analysis was conducted looking at chemotherapy treatment interactions for patients with a RS of 11-25.
The authors reported a statistically significant chemotherapy interaction among patients who were less than 50 years of age: for patients with RS 16-20, there were 9% fewer iDFS events including 2% or less distant recurrences, for those with RS of 21-25, there were 6% fewer iDFS events comprising mainly of distant recurrences.
As the data stands, for patients with node negative hormone receptor positive, HER2 negative breast cancer and a RS of less than 11, there is no role for adjuvant chemotherapy.
study does not truly reflect real world practice as the study included patients with very small tumours with a low clinical risk.
Duration of anti-Her 2 therapy
At ASCO 2017, the data on APHINITY, the addition of pertuzumab to trastuzumab, was presented and showed no OS but disease free survival (DFS) benefit and there was a lot of media coverage and it has changed practice in some revenue driven settings2.
PERSEPHONE, a pivotal non-inferiority trial conducted in the UK was presented at ASCO 2018 looking at the duration of anti-Her 2 therapy – patients were randomised to either 6 months of adjuvant Trastuzumab vs standard 12 months of therapy3.
At a median follow-up of 5.4 years, the trial showed that it had met its primary end-point for DFS (4-y 89.4 vs 89.8%) and secondary end-point for OS (4-y 93.8 vs 94.8%) in non-inferiority.
A pre-defined sub group analysis revealed that groups more likely to benefit from 12 months of trastuzumab therapy compared to 6 months included those patients who received trastuzumab concurrently with chemotherapy, patients who received neoadjuvant chemotherapy, those who received taxanes and among patients who had ER negative disease.
Given the cost-implications of monoclonal antibodies even with the availability of biosimilars, this data does allow some level of confidence in safely recommending 6 months of adjuvant trastuzumab in certain subgroups of patients.
Cardiac toxicity remains an issue and will need to be closely monitored – 4% patients stopped trastuzumab in the 6 mo arm v and 8% in the 12 mo arm (P<0.0001).
Genomics – can it de-escalate therapy?
The precision neoadjuvant therapy trial P-NAT looked at patients with triple-negative breast cancer (TNBC) to see whether molecular profiling improves survival (ARTEMIS)4.
Clinicians were randomised to know or not know (2:1) the molecular profiling data.
The clinicians who did not know the results treated patients with AC (doxorubicin, cyclophosphamide) x 4 followed by taxanes x 4 while the other group received AC x 4 to define chemosensitivity and then received targeted drugs based on response cascade - atezolimumab, panitumumab, enzatulamide, everolimus, mirvetuximab.
Where the clinician did not know the molecular profiling results, the pCR was 50% vs 43% and the pCR/residual cancer burden-1 was 62% vs 56%.
At this point, molecular profiling patients with TNBC does not look promising and treatment continuation based on using standard of care which is clinical and radiologic assessment is recommended.
2. Adjuvant endocrine therapy and predicting late recurrences
There were two studies that looked at ovarian suppression (OvS) in pre-menopausal patients with EBC5,6.
The ASTRRA study compared outcomes between Tamoxifen and OvS for 2 yrs vs tamoxifen alone and showed that adding OvS to Tamoxifen significantly improved DFS.
The study assessed ovarian function longitudinally with FSH levels at 6 monthly intervals for 2 years post end of CT and if the levels were <30 IU/mL or if patients had a menstrual period, they were randomised.
All patients in this study had received chemotherapy so were likely a higher risk group of patients.
The SOFT and TEXT trials have changed practice in that adding OvS to tamoxifen or OvS and exemastane is considered standard of care for premenopausal patients with intermediate or high risk of recurrence.
Regan et al presented the data with a median follow-up of 8-9 years and showed that the improvement in 8-years freedom from distant recurrence relates to the risk of recurrence.
This was done by a very complex composite analysis which is not replicable in the clinics.
Given the data even on patients with T1 cancers (see Table 1 below), predicting late recurrences is an important issue not only to add adjuvant OvS but also to decide upon the duration of endocrine therapy.
There are quite a few tools which are emerging to help with the decision making about late recurrences – and some were presented at ASCO 2018 but of all these, the most promising to be used in clinics currently seems to be the clinical treatment score (CTS5) score - and this includes nodal status, grade, tumour size and age.
CTS5 = 0.438 × nodes 0.988 × (0.093 × size − 0.001 × size2 0.375 × grade 0.017 × age)7.
Patients with high or intermediate score / risk can be considered for 2-5 years of OvS with exemastane / tamoxifen.
Table 1: The risk of late recurrences in patients with hormone receptor positive EBC (More than 50% occur after 5 years) 8-10
Presented by William Gradishar at ASCO 2018
.jpg)
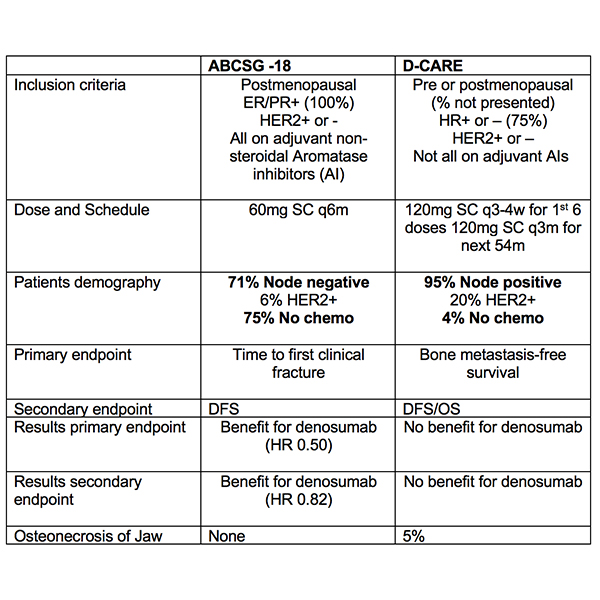
3. Adjuvant bone modifying agents
Both the studies presented on adjuvant use of denosumab (ABCSG-18 and D-CARE) were not practice changing and presented somewhat conflicting information11,12.
Denosumab does prevent bone fractures in early breast cancer patients but at this point denosumab cannot be recommended for improving outcomes- see Table 2 below.
In patients with EBC, adjuvant Zoledronate for 2-3 years remains the standard of care in post-menopausal women with EBC with an intermediate or high risk of disease recurrence based on the AZURE and SUCCESS-A data13,14.
Table 2: comparing the two denosumab trials.
4. Adjuvant Radiotherapy
Internal mammary and medial supraclavicular (IM-MS) lymph nodes
The 15-year results of the randomised EORTC trial 2922/10925 investigating IM-MS radiation in stage I-III breast cancer were presented15.
4,004 patients were randomised to IM-MS radiotherapy (50Gy) vs. no radiotherapy.
The primary end point was OS and at 15-year follow-up, there was no significant improvement in OS though the number of events were quite small in later years.
At 15-years, the survival analysis showed that the patients who had IM-MS radiotherapy had less likelihood of dying of breast cancer and reassuringly there were no increased cardiovascular risks or other cancers .
There was a 3.8% improved breast cancer mortality (hazard ratio of 0.81; P. = 0.0055 ;16% vs. 19.8%) in favour of IM-MS radiotherapy.
The question is whether all eligible patients should get regional nodal irradiation? Given that the patients are having radiotherapy anyway, it is prudent to include the IM-MS group in a selected group of patients.
BRCA 1/2Carriers contralateral breast Radiotherapy
In patients with BRCA 1/2 carriers and breast cancer, contralateral (CL) breast cancer is reduced with older age and oophorectomy; there is data to show that patients with BRCA1/2 carriers, have a higher incidence of CL breast cancer compared to those with sporadic breast cancer (26% vs 3% ; 15% vs 4.4%)16,17 and it has also been shown that there is no evidence of a dose-response relationship with radiotherapy or increase in incidence of CL breast cancer as a result of radiotherapy to the ipsilateral breast.
The study presented at ASCO 2018 was a prospective national trial which recruited 162 BRCA1 /2 carriers, over 30 yrs of age and diagnosed with stage I-III breast cancer between May 2007 and October 2017- of these 81 pts had surgery and radiation to the involved side only (control group) and 81 chose additional radiation to the contralateral uninvolved breast (intervention group)18.
At a median follow up of 60 months, 11.1 % patients developed CL breast cancer compared to 2.5% in the radiotherapy group (P=0.016).
It is postulated that radiotherapy destroys the luminal epithelial compartment which is responsible for BRCA- associated cancers.
This looks to be promising especially in LMIC where the uptake of risk reducing mastectomy is very low and also as a potential solution for high-risk women declining mastectomy though more long-term follow-up is required.
MRI-based breast screening is an option in these women and has proven to be a reasonable alternative to risk-reducing mastectomy but carries its own issues with false positives / unnecessary biopsies and psychological impact19.
5. Survivorship
Lymphoedema
One of the studies looked at the incidence of lymphoedema post neoadjuvant chemotherapy (NACT) as part of a sub study of ACOSOG Z1071 (Alliance for Clinical Trials in Oncology) trial for patients with node-positive breast cancer20.
The study included 486 patients and looked at the measures at 3 years - volume 10% increase, 20% increase and patient reported swelling or heaviness.
The study showed that lymphoedema was high in patients with obesity (BMI > 30, p = 0.02) and in those with the duration of NACT being > 144 days (p = 0.029).
Severe lymphoedema was also high with longer duration of NACT (p = 0.01).
On multivariate analysis, obesity and length of NACT remained significant variables.
As we are moving towards using more NACT especially in TNBC and Her 2 positive cancers, this study becomes relevant especially in node positive patients with larger tumours which reflects the study population.
More and more centres that would use 6 cycles of NACT (126 days) are now moving towards 8 cycles of chemotherapy (AC x 4 followed by TPH x 4 or AC x 4 followed by weekly Pacli /-carbo x 12 weeks = 168 days).
We need to be mindful of the long-term lymphoedema rate in outpatient department for those patients who have received longer duration of NACT.
Arthralgia and aromatase inhibitors
Arthralgia remains a significant toxicity of adjuvant endocrine treatment in EBC and is one of the common reasons for non-compliance.
In this interesting study21, patients with an aromatase inhibitor and a Brief Pain Inventory (BPI) score of >5 /10 were (n=249) were randomised to Omega-3 fatty acid 3.3 g/day for 24 weeks and placebo.
Pain scores and BPI was assessed at baseline, 6, 12, and 24 weeks.
The results showed that there was a significant decrease in BPI worst pain from baseline for both Omega-3 and placebo groups though there were no significant differences between the two, but when these results were looked at with the BMI score, there was a significant improvement in pain and for patients who had a BMI of >30, the worst pain, average pain, and pain interference scores were better with omega-3 fatty acid.
It can be concluded that in obese patients, BPI scores after 24 weeks of omega-3 Fatty acid treatment were significantly lower and also the joint specific symptoms were significantly lower at 24 weeks on those patients who received the omega-3 fatty acid.
These drugs can easily be recommended in the outpatient setting –they are relatively low cost, have a good safety profile and also have a positive impact on the triglycerides, another lipid profile component.
References
1. Sparano JA, Gray RJ, Wood WC, et al. TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score. J Clin Oncol 36, 2018 (suppl; abstr LBA1)
2. von Minckwitz G, Procter M, de Azambuja E, et al: Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377:122-131, 2017.
3. Earl HM, Hiller L, Vallier A-L, et al. PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive ( ) early breast cancer (EBC): Randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results. J Clin Oncol 36, 2018 (suppl; abstr 506)
4. Moulder SL, Hess KR, Candelaria RP et al. Precision neoadjuvant therapy (P-NAT): A planned interim analysis of a randomized, TNBC enrolling trial to confirm molecular profiling improves survival (ARTEMIS). J Clin Oncol 36, 2018 (suppl; abstr 518)
5. Noh WC, Lee JW, Nam SJ, et al. Role of adding ovarian function suppression to tamoxifen in young women with hormone-sensitive breast cancer who remain premenopausal or resume menstruation after chemotherapy: The ASTRRA study. J Clin Oncol 36, 2018 (suppl; abstr 502)
6. Regan MM, Francis PA, Pagani O et al Absolute improvements in freedom from distant recurrence with adjuvant endocrine therapies for premenopausal women with hormone receptor-positive (HR ) HER2-negative breast cancer (BC): Results from TEXT and SOFT. J Clin Oncol 36, 2018 (suppl; abstr 503)
7. Dowsett M, Sestak I, Regan MM et al. Integration of Clinical Variables for the Prediction of Late Distant Recurrence in Patients With Estrogen Receptor-Positive Breast Cancer Treated With 5 Years of Endocrine Therapy: CTS5. J Clin Oncol. 2018 Jul 1;36(19):1941-1948.
8. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 May 14-20;365(9472):1687-717.
9. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996 Oct;14(10):2738-46.
10. Pan H, Gray RG, Davies C, et al. Predictors of recurrence during years 5-14 in 46,138 women with ER breast cancer allocated 5 years only of endocrine therapy (ET). J CLin Oncol, 2016;34 (15):505a.
11. Gnant M, Pfeiler G, Steger GG et al. Adjuvant denosumab in early breast cancer: Disease-free survival analysis of 3,425 postmenopausal patients in the ABCSG-18 trial. J Clin Oncol 36, 2018 (suppl; abstr 500)
12. Coleman RE, Finkelstein D, Barrios CH et al. Adjuvant denosumab in early breast cancer: First results from the international multicenter randomized phase III placebo controlled D-CARE study. J Clin Oncol 36, 2018 (suppl; abstr 501)
13. Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014 Aug;15(9):997-1006
14. Janni W, et al: Extended adjuvant bisphosphonate treatment over five years in early breast cancer does not improve disease-free and overall survival compared to two years of treatment. 2017 San Antonio Breast Cancer Symposium. Abstract GS1-06. Presented December 6, 2017.
15. Poortmans P, Collette S, Struikmans H, et al. Fifteen-year results of the randomised EORTC trial 22922/10925 investigating internal mammary and medial supraclavicular (IM-MS) lymph node irradiation in stage I-III breast cancer. J Clin Oncol 36, 2018 (suppl; abstr 504)
16. Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006 Jun 1;24(16):2437-43. JCO 2006;
17. Bernstein JL, Thomas DC, Shore RE et al , Contralateral breast cancer after radiotherapy among BRCA1 and BRCA2 mutation carriers: a WECARE study report. Eur J Cancer. 2013 Sep;49(14):2979-85
18. Evron E, David MAB, Goldberg H, et al. Phase II national clinical trial of prophylactic irradiation to the contralateral breast for BRCA mutation carriers treated for early breast cancer (EBC). J Clin Oncol 36, 2018 (suppl; abstr 514)
19. Warner E, Zhu S, Hill K et al. "What if I keep my breasts?" Extended follow-up of unaffected BRCA mutation carriers diagnosed with breast cancer (BC) in the Toronto magnetic resonance imaging (MRI) screening study.J Clin Oncol 36, 2018 (suppl; abstr 1523)
20. Boughey JC, Ballman KV, McCall LM, et al. Factors associated with lymphedema in patients/women with node positive breast cancer treated with neoadjuvant chemotherapy and axillary dissection on a prospective clinical trial. J Clin Oncol 36, 2018 (suppl; abstr 513)
21. Shen S, Unger JM, Crew KD et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927).
J Clin Oncol 36, 2018 (suppl; abstr 10000)
Address for Correspondence
Dr Bhawna Sirohi
Consultant Medical Oncologist
Barts Health NHS Trust
London EC1A 7BE
Tel: 44 7468520613 | Email: bhawna.sirohi13@gmail.com
The World Cancer Declaration recognises that to make major reductions in premature deaths, innovative education and training opportunities for healthcare workers in all disciplines of cancer control need to improve significantly.
ecancer plays a critical part in improving access to education for medical professionals.
Every day we help doctors, nurses, patients and their advocates to further their knowledge and improve the quality of care. Please make a donation to support our ongoing work.
Thank you for your support.