Efficacy of lenalidomide in association with cyclophosphamide and dexamethasone in multiple myeloma patient with bilateral retro-orbital localisation
S Felici 1, N Villivà 1, G Balsamo 2 and A Andriani 1
1 Haematology Unit, Nuovo Regina Margherita, Hospital, 00153 Rome, Italy
2 Histopathology Complex Unit, Santo Spirito Hospital, 00193 Rome, Italy
Correspondence to: Stefano Felici. Email: sfelici51@gmail.com
Abstract
Extramedullary localisation is an uncommon manifestation in multiple myeloma (MM). Ocular involvement is rare. Here, we describe a relapse of MM with bilateral retro-orbital localisation without any bone involvement with good and rapid response to therapy with lenalidomide, dexamethasone, and cyclophosphamide.
Keywords: multiple myeloma, extramedullary disease, ocular involvement, lenalidomide.
Introduction
Multiple myeloma (MM) accounts for 1% of malignant diseases and for 10% of haematologic malignancies [1].
It is a clonal B cell tumour characterised by monoclonal protein production and by the slow proliferation of plasma cells, mainly in bone marrow.
Marrow infiltration by plasma cells, monoclonal protein production, and the reaction of the bone marrow microenvironment are the causes of lytic bone lesions, anaemia (with or without thrombocytopenia and leucopenia), loss of normal immune response, renal failure, and hypercalcaemia.
According to the International Myeloma Working Group, to make a diagnosis of MM it is necessary to demonstrate the presence of at least 10% plasma cells on bone marrow examination or on extramedullar tissue biopsy, as well as monoclonal protein (in serum or urine) and organ damage [2].
The acronym CRAB defines organ damage: hypercalcaemia, renal failure, anaemia, bony lesions [3–5].
Solitary plasmacytoma of the bone represents 3%–5% of plasma cell dyscrasias. In addition to finding a tumour composed of light chain restricted plasma cells on biopsy, in this case it is necessary to demonstrate the presence of a normal bone marrow and the absence of other skeletal lesions [through magnetic resonance imaging (MRI), positron emission tomography–computed tomography (PET–CT) scans], and the presence of monoclonal protein in serum and urine. Almost 50% of patients with plasmacytoma can develop an MM [6].
Extramedullary plasmacytoma can also occur in soft tissue. It is more frequent in the tonsils, nasopharynx, gastrointestinal tract, and paranasal sinuses. To identify it correctly, the absence of bony lesions and plasma cell infiltration in bone marrow must be demonstrated. Monoclonal protein can be present. Radiotherapy is the treatment of choice associated—if necessary—with surgical resection [7].
Extramedullary disease (EMD) is a clinical manifestation of myeloma. Bladè et al reported that the incidence of EMD in newly diagnosed myeloma ranges from 7% to 18%; 6% to 20% of patients can develop EMD at a later stage of the disease [8].
EMD can arise from the destruction of cortical bone at the starting point of a tumour or from haematogenous metastatic spread [8]. Local growth after the destruction of cortical bone is the most frequent. Haematogenous spread is more frequent in the skin, liver, kidneys, or in the nervous system.
Ophthalmic involvement is rare in MM and can damage any structure of the eye [4, 9].
Here, we describe the case of a relapsed MM patient with bilateral retro-orbital localisation without any bone involvement who was treated with lenalidomide, dexamethasone and cyclophosphamide.
Case report
In February 2010, a 73-year-old male patient arrived at our institution because of anaemia, which was diagnosed in January 2010 after laboratory tests were performed to investigate the origin of thoracic pain. His haemoglobin levels were 8.5 gr/dl, and the other blood tests showed moderate renal failure and the presence of a monoclonal component IgG (K). The skeletal X-ray was negative with regard to the presence of osteolysis, and the bone marrow biopsy showed plasma cell infiltration of about 50%.
Symptomatic MM was diagnosed, and a treatment with bortezomib (Velcade) biweekly (days 1, 4, 8, 11, 22, 25, 28, 32) Melphalan and Prednisone from day 1 to day 4, every 35 days (VMP) was started.
The chemotherapy, combined with diuretic treatment and erythropoietin support (epoetin alfa 40,000 units subcutaneous weekly), was completed after four cycles in August 2010. The patient achieved a subjective improvement with a documented recovery of the renal function as well as an improvement of anaemia and the disappearance of dorsal pain.
Even though the partial remission was documented by the reduction of the monoclonal component, to 1 gr/dl, the patient presented neurological symptoms characterised by difficulty in walking and diplopia due to the paralysis of the oculomotor nerve. Before the fifth cycle of VMP, he was admitted to the Department of Neurology, where a bortezomib-related neuropathy was diagnosed [52].
During hospitalisation, the patient obtained a partial recovery of ambulation and therefore a specific treatment for MM—only including melphalan and prednisone—was restarted after discharge and continued for another four cycles. In November 2010, distal oedemas associated with hyponatraemia and hypoalbuminaemia appeared, and treatment with diuretics and albumin was started. The IgG blood levels became <1 g/dl, and the bone marrow aspiration showed a reduction of bone-marrow plasma cells up to 1%–2% of all cellularity as well as red cells progenitor hyperplasia. Periumbilical fat aspiration was negative to Congo red stain.
Maintenance with monthly dexamethasone and zoledronic acid was performed.
In August 2011, the patient came to our Day Hospital with bilateral exophthalmos (left > right), periorbital oedema, conjunctival hyperaemia, diplopia, continuous pain, and decreased vision (Figures 1 and 2).
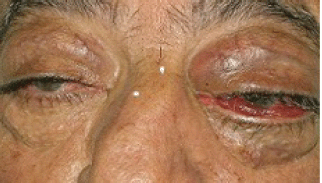
Figure 1: Bilateral exophthalmos (left > right), periorbital oedema, conjunctival hyperaemia.
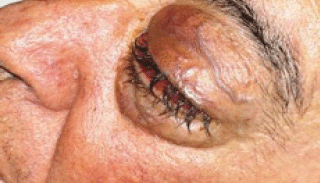
Figure 2: Detail of left eye.
An MRI scan of the central nervous system showed inspissations of the extrinsic ocular muscles and especially of the left medial rectus muscle. A biopsy was proposed but could not be performed due to technical problems.
At this point, to reduce the continuous pain and the decreased vision, we started treatment with cyclophosphamide (500 mg/w i.v. for three consecutive weeks and one of suspension), lenalidomide [10 mg/daily orally days 1–21 every 28 days], and dexamethasone (40 mg intravenously, days 1–2, 8–9, 15–16) (L-CD) [6, 7].
After the first cycle, the exophthalmos, periorbital oedema, conjunctival hyperaemia, diplopia, and continuous pain disappeared (Figures 3 and 4).
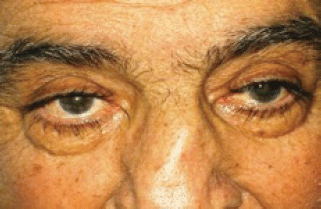
Figure 3: The exophthalmos, periorbital oedema, conjunctival hyperaemia disappeared after first cycle of therapy.
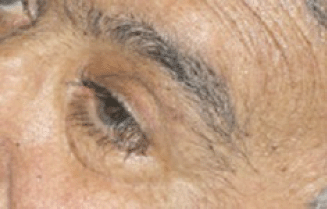
Figure 4: Detail of left eye after first cycle of therapy.
Treatment was modified at the third cycle for better patient compliance, and cyclophosphamide (50 mg orally days 1–21) in combination with lenalidomide was prescribed for three more cycles.
Until April 2012, the patient continued treatment and maintained complete response but unfortunately, in May 2012, the patient died due to cardiac arrest.
Discussion
MM may cause ocular pathologies by direct infiltration or as an EMD. This results in the displacement or compression of tissues, by causing hyperviscosity syndrome and by immunoglobulin light chain deposition in ocular tissues. Rarely, ocular findings may be the first manifestation of the disease; more frequently they may occur as one of the extramedullary manifestations of the disease and as the sign of insufficient chemotherapy or a relapse [10].
In the retina, cotton-wool spots, nerve fibre dropout, haemorrhages, and vascular abnormalities (including dilatation, tortuosity, and microaneurysms) can develop secondary to the associated serum hyperviscosity. Optic nerve, oculomotor nerve and lacrimal gland infiltration by myeloma cells have been reported. Iridescent crystalline or copper deposits in the conjunctiva and cornea, ciliary body cysts, and cranial nerve compression causing palsies have also been described [9].
Orbital involvement occurs more frequently than intraocular involvement and is typically diagnosed through CT [10, 11].
In the past, several authors reported the occurrence of ocular manifestations in MM [12–30].
In 2011, Chin et al performed a revue of the literature, presenting three cases of ocular lesions in MM [9]: Sanders et al in 1967 [13], reported the occurrence of ocular lesions in 12/15 eyes (80%) examined in autopsy studies and showed that these localisations are more frequent than clinically noted.
In 1972, Rodman and Font described 30 cases with ocular lesions associated with other signs of systemic disease [14].
Burkat et al in 2009 reviewed 52 cases reported in 41 articles; of these, 88% presented a unilateral lesion, 81% proptosis, 23% decreased vision, 23% diplopia, 21% swelling, and 13% ptosis [28].
If ocular lesions are suspected, a CT (or MRI or PET-CT) scan must be performed as well as, if possible, a biopsy of the lesion to demonstrate the presence of plasma cells CD 56, CD 38 and CD 138 positive, CD 20 negative.
The treatment of MM evolved substantially over the last decade, most notably with the introduction of highly effective novel agents (thalidomide, lenalidomide and bortezomib) the use of which resulted in considerable improvements in the outcome [31–35].
Lenalidomide has immunomodulatory effects, anti-inflammatory, antiangiogenic and proapoptotic activities; lenalidomide has direct effects on tumour cells, the tumour microenvironment, cytokine responses, production of growth factors, and T cell activation [40–43].
Lenalidomide combined with dexamethasone is an effective treatment for patients with relapsed/refractory MM and is associated with increased response rates and prolonged progression-free survival and overall survival (OS) compared with dexamethasone alone [36, 37, 49, 50]. The Len Dex regimen was effective regardless of the type of prior therapy received [44, 45]. Moreover Len Dex was found to be effective when given at first relapse [46]. Continued treatment led to greater depth of response and improved survival outcomes [47, 48]. The addition of cyclophosphamide appeared to enhance the efficacy of Len Dex, suggesting that such combination therapy may be used more in clinical practice [38, 39]. Furthermore, lenalidomide and dexamethasone are effective for EMD in refractory or relapsed myeloma (overall response rate of 61.1% with compete disappearance in 44.1%) [51].
Conclusion
Our patient successfully went through a first-line therapy based on bortezomib, melphalan and dexamethasone (four cycles), resulting in a partial response. However, this treatment had to be stopped following the development of a severe neuropathy. The melphalan/prednisonebased treatment (in combination with dexamethasone and zoledronic acid) was successfully continued, resulting in a very good partial response up to four months before the relapse.
When the extramedullary relapse the percentage of bone-marrow plasma cells was <5%, with monoclonal IgG < 1 g/dl. Therefore, we decided to start a new combination of treatment, based on lenalidomide. To attain a quicker response, this treatment was supplemented with dexamethasone and cyclophosphamide.
Efficacy of new biologic treatments on extraosseous (ocular) localisations of MM is a highly debated question. To date, only a few case reports have been described.
We report this case due to the rapid response to the therapy of the retro-orbital localisation.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
1 Tricot G (2009) Multiple Myeloma in Hematology, Basic Principles and Practice 5th edn ed Hoffman et al (Philadelphia: Churchill Livingstone Elsevier) ch 87 1387
2 International myeloma working group (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the international myeloma working group Br J Haematol 121 749–57. PMID: 12780789
3 Bladé J et al (2011) Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach J Clin Oncol 29(28) 3805–12. DOI: 10.1200/JCO.2011.34.9290 PMID: 21900099
4 Varettoni M et al (2009) Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1,003 consecutive patients Ann Oncol 21(2) 325–30. DOI: 10.1093/annonc/mdp329 PMID: 19633044
5 Wu P et al (2009) The impact of extramedullary disease at presentation in outcome of myeloma Leuk Lymphoma 50(2) 230–5. DOI: 10.1080/10428190802657751 PMID: 19197724
6 Tricot G (2009) Multiple Myeloma in Hematology, Basic Principles and Practice 5th edn ed Hoffman et al (Philadelphia: Churchill Livingstone Elsevier) ch 87 1400
7 Bladè J et al (2011) Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach J Clin Oncol 29(28) 3805–12. DOI: 10.1200/JCO.2011.34.9290 PMID: 21900099
8 Bladè J et al (2012) Extramedullary involvement in multiple myeloma Haematologica 97(11) 1618–19. DOI: 10.3324/haematol.2012.078519 PMID: 23125242 PMCID: 3487431
9 Chin KJ et al (2011) Ocular manifestations in multiple myeloma: three cases and a review of the literature Optometry 82 224–30. DOI: 10.1016/j.optm.2010.10.009 PMID: 21193351
10 Fung S et al (2005) Ophthalmic manifestations of multiple myeloma Ophthalmologica 219(1) 43–8. DOI: 10.1159/000081782 PMID: 15627827
11 Bataille R, Harousseau JL (1997) Multiple myeloma N Engl J Med 336(23) 1657–64. DOI: 10.1056/NEJM199706053362307 PMID: 9171069
12 Omoti AE et al (2007) Ophthalmic manifestations of multiple myeloma West Afr J Med 26(4) 265–8. PMID: 18705423
13 Sanders TE, Podos SM, Rosenbaum LJ (1967) Intraocular manifestations of multiple myeloma Arch Ophthalmol 77(6) 789–94. DOI: 10.1001/archopht.1967.00980020791015 PMID: 6026189
14 Rodman HI et al (1972) Orbital involvement in multiple myeloma. Review of the literature and report of three cases Arch Ophthalmol 87 30–5. DOI: 10.1001/archopht.1972.01000020032006 PMID: 4550269
15 Knapp AJ et al (1987) Multiple myeloma and its ocular manifestations Surv Ophthalmol 5 343–51. DOI: 10.1016/0039-6257(87)90119-6
16 Sharma S et al (2006) Cytodiagnosis of multiple myeloma presenting as orbital involvement: a case report CytoJournal 3 19. DOI: 10.1186/1742-6413-3-19 PMID: 16901345 PMCID: 1564147
17 Adkins JW, Shields JA, Shields CL (1997) Plasmacytoma of the eye and orbit Int Ophthalmol 20 339–43. DOI: 10.1007/BF00176888
18 Rootman J et al (2003) Lymphoproliferative, Leukemia, and Histiocytic Lesions of the Orbit, ed Rootman J Disease of the Orbit (Philadelphia: Lippincott Williams and Wilkins) pp. 402–6.
19 Howling SJ et al (1998) Case report: the ct features of orbital multiple myeloma Clin Radiol 53 304–5. DOI: 10.1016/S0009-9260(98)80133-5 PMID: 9585050
20 Munshi NC, Anderson KC (2005) Plasma Cell Neoplasms, ed Devita VT, Hellman S and Rosenberg SA Cancer: Principles and Practice of Oncology 7th edn (Philadelphia: Lippincott Williams and Wilkins) pp. 2155–85.
21 Shields CL et al (2007) Sequential bilateral solitary extramedullary plasmacytoma of the ciliary body Cornea 26(6) 759–61. DOI: 10.1097/ICO.0b013e3180621087 PMID: 17592334
22 Tung G et al (1988) Plasmacytoma of the orbit Arch Ophthalmol 106:1622–35. DOI: 10.1001/archopht.1988.01060140790056 PMID: 3190549
23 Ezra E et al (1995) Inadequately irradiated solitary extramedullary plasmacytoma of the orbit requiring exenteration Am J Ophthalmol 120(6) 803–5. PMID: 8540559
24 Aronson SA, Shaw R (1959) Corneal crystals in multiple myeloma Arch Ophthalmol 61 541–6. DOI: 10.1001/archopht.1959.00940090543007
25 Shen YC, Wang CY, Huang TY (2006) Multiple myeloma manifesting as a salmon patch conjunctival mass Am J Ophthalmol 474 948–9. DOI: 10.1016/j.ajo.2005.11.059
26 Franklin RM, Kenyon KR, Green WR (1982) Epibulbar IgA plasmacytoma occurring in multiple myeloma Arch Ophthalmol 100 451–6. DOI: 10.1001/archopht.1982.01030030453015 PMID: 7065966
27 Shakin EP, Augsburger JJ, Eagle RC (1988) Multiple myeloma involving the iris Arch Ophthalmol 106 524–6. DOI: 10.1001/archopht.1988.01060130570039 PMID: 3355422
28 Burkat CN, Van Buren JJ, Lucarelli MJ (2009) Characteristics of orbital multiple myeloma: a case report and literature review Surv Ophthalmol 54 697–704. DOI: 10.1016/j.survophthal.2009.04.012 PMID: 19709708
29 Fung S et al (2005) Ophthalmic manifestations of multiple myeloma Ophthalmologica 219(1) 43–8. DOI: 10.1159/000081782 PMID: 15627827
30 Khouri GG et al (1986) Clinicopathologic features in two cases of multiple myeloma Retina 6(3) 169–75. DOI: 10.1097/00006982-198600630-00007 PMID: 3099353
31 Kumar SK et al (2008) Improved survival in multiple myeloma and the impact of novel therapies Blood 111 2516–2520. DOI: 10.1182/blood-2007-10-116129
32 Brenner H, Gondos A, Pulte D (2008) Recent major improvement in long-term survival of younger patients with multiple myeloma Blood 111 2521–2526. DOI: 10.1182/blood-2007-08-104984
33 Kristinsson SY et al (2007) Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in sweden from 1973 to 2003 J Clin Oncol 25 1993–1999. DOI: 10.1200/JCO.2006.09.0100 PMID: 17420512
34 Kastritis E et al (2009) Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the international staging system (ISS): an analysis of the greek myeloma study group (GMSG) Leukemia 23 1152–1157. DOI: 10.1038/leu.2008.402 PMID: 19225533
35 Kumar SK et al (2012) Risk of progression and survival in multiple myeloma relapsing after therapy with imids and bortezomib: a multicenter international myeloma working group study Leukemia 26 149–157. DOI: 10.1038/leu.2011.196
36 Dimopoulos M et al (2007) Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma N Engl J Med 357 2123–32. DOI: 10.1056/NEJMoa070594 PMID: 18032762
37 Weber DM et al (2007) Lenalidomide plus dexamethasone for relapsed multiple myeloma in north america N Engl J Med 357 2133–42. DOI: 10.1056/NEJMoa070596 PMID: 18032763
38 Morgan GJ et al (2007) Lenalidomide in combination with cyclophosphamide and dexamethasone is an effective and tolerated regimen for myeloma patients Br J Haematol 137 268–9. DOI: 10.1111/j.1365-2141.2007.06538.x PMID: 17408469
39 Schey SA et al (2010) The addition of cyclophosphamide to lenalidomide and dexamethasone in multiply relapsed/refractory myeloma patients: a phase I/II study Br J Haematol 150 326–33. DOI: 10.1111/j.1365-2141.2010.08250.x PMID: 20553268
40 Palumbo A et al (2009) International myeloma working group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation Leukemia 23 1716–30. DOI: 10.1038/leu.2009.122 PMID: 19494840
41 Kotla V et al (2009) Mechanism of action of lenalidomide in hematological malignancies J Hematol Oncol 2 36. DOI: 10.1186/1756-8722-2-36 PMID: 19674465 PMCID: 2736171
42 Richardson PG et al (2002) Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma Blood 100 3063–7. DOI: 10.1182/blood-2002-03-0996 PMID: 12384400
43 Richardson PG et al (2006) A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma Blood 108 3458–64. DOI: 10.1182/blood-2006-04-015909 PMID: 16840727 PMCID: 1895441
44 Wang M et al (2008) Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure Blood 112(12) 4445–51. DOI: 10.1182/blood-2008-02-141614 PMID: 18799726
45 Chanan-Khan AA et al (2006) Lenalidomide (L) in combination with dexamethasone (D) significantly improves time to progression (TTP) in non-stem cell transplant patients (pts) with relapsed or refractory (rel/ref) multiple myeloma (MM): analysis from MM-009 and MM-010 randomized phase III clinical trials Blood 108 Abstract 3554.
46 Stadtmauer EA et al (2009) Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma Eur J Haematol 82 426–32. DOI: 10.1111/j.1600-0609.2009.01257.x PMID: 19302559 PMCID: 2704925
47 San-Miguel JF et al (2011) Effects of lenalidomide and dexamethasone treatment duration on survival in patients with relapsed or refractory multiple myeloma treated with lenalidomide and dexamethasone Clin Lymphoma Myeloma Leuk 11 38–43. DOI: 10.3816/CLML.2010.n.120 PMID: 21273172
48 Harousseau JL et al (2010) Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma Haematologica 95 1738–44. DOI: 10.3324/haematol.2009.015917 PMID: 20460639 PMCID: 2948100
49 Gozzetti A et al (2009) Central Nervous System and Intracranial Myeloma: A Retrospective Italian Multicenter Study American Society of Hematology 51st Annual Meeting and Exposition (New Orleans, LA, 5–8 Dec.) Abstract # 1882.
50 Warren KE et al (2011) Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary cns tumors: pediatric brain tumor consortium study PBTC-018 J Clin Oncol 29(3) 324–9. DOI: 10.1200/JCO.2010.31.3601 PMCID: 3056466
51 Calvo-Villas JM et al On behalf of the GEM-PETHEMA/Spanish Myeloma Group, Spain (2011) Lenalidomide is effective for extramedullary disease in relapsed or refractory multiple myeloma Eur J Haematol 87(3) 281–84. DOI: 10.1111/j.1600-0609.2011.01644.x PMID: 21557775
52 Argyriou AA et al (2008) Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature Blood 112 1593–99. DOI: 10.1182/blood-2008-04-149385 PMID: 18574024






