Repurposing Drugs in Oncology (ReDO)—itraconazole as an anti-cancer agent
Pan Pantziarka1,2, Vidula Sukhatme3, Gauthier Bouche1, Lydie Meheus1 and Vikas P Sukhatme3,4
1Anticancer Fund, 1853 Strombeek-Bever, Belgium
2The George Pantziarka TP53 Trust, London, KT1 2JP, UK
3GlobalCures, Inc; Newton MA 02459, USA
4Beth Israel Deaconess Medical Centre and Harvard Medical School, Boston, MA 02215, USA
Correspondence to: Pan Pantziarka. Email: pan.pantziarka@anticancerfund.org
Abstract
Itraconazole, a common triazole anti-fungal drug in widespread clinical use, has evidence of clinical activity that is of interest in oncology. There is evidence that at the clinically relevant doses, itraconazole has potent anti-angiogenic activity, and that it can inhibit the Hedgehog signalling pathway and may also induce autophagic growth arrest. The evidence for these anticancer effects, in vitro, in vivo, and clinical are summarised, and the putative mechanisms of their action outlined. Clinical trials have shown that patients with prostate, lung, and basal cell carcinoma have benefited from treatment with itraconazole, and there are additional reports of activity in leukaemia, ovarian, breast, and pancreatic cancers. Given the evidence presented, a case is made that itraconazole warrants further clinical investigation as an anti- cancer agent. Additionally, based on the properties summarised previously, it is proposed that itraconazole may synergise with a range of other drugs to enhance the anti-cancer effect, and some of these possible combinations are presented in the supplementary materials accompanying this paper.
Keywords: drug repurposing, itraconazole, hedgehog pathway inhibition, ReDO project
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Current usage
Introduction
Itraconazole (ITZ) is a triazole anti-fungal treatment widely used in the prevention and systemic treatment of a broad range of fungal infections, including aspergillosis, blastomycosis, candidiasis, histoplasmosis, and in some dermatological and nail infections. The mechanism of action for this antifungal activity is through the decrease of ergosterol synthesis, required for membrane integrity of fungal cells, via inhibition of the lanosterol 14 alpha-demethylase (14DM) catalyst. Immunocompromised patients are often treated prophylactically with triazole anti-fungal drugs, including ITZ, particularly if there is a risk of aspergillosis. ITZ is commonly available as a generic, prescription-only drug. Common trade names include Sporanox (Janssen) and Onmel (Merz).
Dosage
ITZ is most commonly administered orally, either as 100 mg or 200 mg capsules or as oral solution. It can also be administered intravenously, though this route is less commonly used. Dosing varies by indication; generally it is used in the range 100 mg–600 mg daily, for between one to 30 days. It is also used for long-term maintenance or prophylaxis, for example 200 mg–400 mg daily for HIV-infected patients, 400 mg daily for patients suffering from chronic pulmonary aspergillosis, or 100 mg per day for more than one year for the treatment of paracoccidioidomycosis [1].
Toxicity
ITZ is generally well-tolerated, though caution is advised with patients at high risk of heart failure or impaired hepatic function. The most common side effects of ITZ are nausea, abdominal pain, and rash. Less commonly, gastrointestinal upsets have been reported, (including vomiting, flatulence, diarrhoea, and constipation), headache, dizziness, and peripheral neuropathy. Rare but serious side effects have included liver failure, chronic heart failure, and neutropenia. ITZ is contra-indicated during pregnancy unless the risk of fungal infection is life-threatening; it is also not advised during breast feeding [2]. Doses are reduced for children, being in the range of 5–10 mg/kg/day, although for severe infections the maximum doses in children are equivalent to the adult doses (for example 400 mg/day for one year for the treatment of severe blastomycosis in children) [3].
Pharmacokinetics
Bioavailability of ITZ is maximised by taking with food for the encapsulated form, or on an empty stomach for the oral solution. A single 200 mg dose, as a capsule with food, produces an average peak plasma concentration of 239 ng/mL (0.34μM) within 4.5 hours, whereas at steady state (after 14 days of 200 mg every 12 hours), the average plasma concentration is 1881 ng/mL (2.67 μM) [4]. While peak plasma concentrations and elimination half-life are similar with the capsule form, the oral solution has approximately 30% greater overall bioavailability as measured by area under the curve (AUC) [5]. The plasma half-life of 200 mg of the capsule form is 24 hours at steady state. The mean absolute bioavailability is around 55%, and as a highly lipophilic molecule ITZ has a high affinity for tissues, achieving concentrations two to ten times higher than those in plasma [6, 7].Oral absorption of ITZ is reduced when gastric acid production is decreased, thereby caution is advised for patients taking H2 receptor agonists (H2RAs) (e.g. cimetidine or ranitidine) or proton pump inhibitors (e.g. omeprazole or pantoprazole).
ITZ undergoes extensive first-pass hepatic metabolism, with unchanged drug barely detectable in urine and between 3–18% of the dose given detectable as the parent drug in the faeces of healthy volunteers. Hydroxyitraconazole also has anti-fungal activity [8]. ITZ is a potent inhibitor of cytochrome P450 (CYP) 3A4, and there is an extensive list with regard to drug interactions [9]. In the context of cancer treatment, it should be noted that there are a number of credible case reports that concomitant ITZ increases the severity of neurotoxicity associated with vincristine [10, 11].
Overall there is a high-degree of inter and intra-patient variability in plasma concentrations, with differences influenced by drug formulation (oral solution or tablets), changes in kinetics during long-term treatments, interactions with food intake, and other medications and changes in pathological status of patients. It is generally recommended, therefore, that for long-term treatment patients be regularly monitored for plasma levels [6, 12, 13].
Pre-clinical evidence in cancer—in vitro and in vivo
Early pre-clinical investigation of the anticancer potential of ITZ focused on a potential role as a potentiator for chemotherapeutic drugs, particularly as a possible agent to reverse multi-drug resistance (MDR).Examples include in vitro studies on the reversal of MDR in a murine P388 leukaemia cell line resistant to daunorubicin [14], human leukaemia cell lines resistant to Adriamycin and etoposide [15] and in human breast cancer resistance protein (BCRP) expressing human embryonic kidney (HEK) cells resistant to topotecan [16]. Of note these results were achieved using clinically achievable doses of ITZ, a finding that was later confirmed in clinical studies (see next section).
In addition to reversing MDR action, other pre-clinical studies have indicated that ITZ has a range of activities of value in an anti-cancer context. A drug screen of a large panel of FDA-approved drugs in a human umbilical vein endothelial cell (HUVEC) proliferation assay indicated that ITZ had potent anti-angiogenic activity [17]. The in vitro screen was confirmed in vivo using a murine Matrigel model, which showed that the mice treated with an IV dose of ITZ equivalent to a typical human dose showed a 67.5% decrease in new vessel formation compared to control mice. Analysis showed that the levels required for an anti-angiogenic response could also be achieved using a 200 mg oral dose of the drug.
Subsequently, the same research group performed a screen for repurposed drug combinations with anti-angiogenic activity. This screen of 741 drug combinations identified ITZ and the immunosuppressant cyclosporin A as a potent and synergistic anti-angiogenic combination [18].
The anti-angiogenic activity of ITZ was later investigated in a panel of non-small cell lung cancer (NSCLC) cell lines (NCI-H358, NCIH1838, NCI-H596, and NCI-H1975) and two primary NSCLC xenograft models [19]. ITZ was shown to inhibit proliferation of HUVEC, but there was no evidence of a direct anti-proliferative effect on NSCLC cells. ITZ, alone and in combination with cisplatin, inhibited growth of primary NSCLC xenografts, increased expression of HIF1α, and reduced tumour vascular area compared to vehicle-treated controls.
A drug screen also identified ITZ as an inhibitor of the Hedgehog pathway at a clinically relevant concentration of 800 nM [20]. Interestingly, the primary metabolite (hydroxyitraconazole), was also shown to be an inhibitor of Hedgehog signalling. In addition to in vitro analysis, an in vivo model of murine Hedgehog-dependent medulloblastoma showed that ITZ treatment at 100 mg/kg b.i.d. or 75 mg/kg b.i.d., both reduced tumour growth. The same study also used a murine basal cell carcinoma (BCC) model, and treatment with ITZ suppressed tumour growth. When treatment was interrupted BCC tumours recommenced growth. The authors estimated that the human doses of ITZ required to achieve the serum levels achieved in these murine models is in the range of 600–900 mg/day, a high dose but one which has been used clinically for long periods in humans [21].
Further work by the same group has shown that ITZ, along with arsenic trioxide (ATO), is able to reverse resistance to existing Hedgehog inhibitors being tested in clinical trials (the cyclopamine analogue IPI-926 and cyclopamine competitive binding agents such as vismodegib, NVP-LDE225, and XL-139) [22]. Using BCC and medulloblastoma mouse allograft models resistant to mutant Hedgehog signalling, the authors showed that ITZ and ATO either singly and in combination were effective against resistant tumours.
Hedgehog signalling was also investigated in a panel of eight malignant pleural mesothelioma (MPM) cell lines in vitro [23]. After showing that Hedgehog signalling was upregulated in the cell lines, the authors tested four pathway inhibitors: vismodegib, ITZ, ATO, and the investigational agent GANT61. Results showed that ITZ was effective in all eight cell lines with IC50< 5 μM.
Investigation into the anti-cancer effect in glioblastoma has found that ITZ treatment, both in vitro and in vivo, causes dose dependent growth arrest but not apoptosis in U87 and C6 glioblastoma cells and a xenograft mouse model [24]. Analysis shows that this growth inhibition is related to induction of autophagy, and that the inhibition of autophagy reverses the effect of ITZ. Induction of autophagy is shown to be related to inhibition of the AKT-mTOR pathway, possibly related to ITZ-induced changes in cholesterol trafficking.
Evidence has also been produced that indicates that clinicians may need to exercise caution in the use of ITZ therapy in patients being treated with monoclonal antibodies. The monoclonal antibody rituximab is an effective therapy for diseases characterised by CD20-expressing B cell dysfunction or over-expression, including lymphomas, leukaemias, and auto-immune conditions. Concurrent use of ITZ was shown, in vitro and in vivo using a murine xenograft model of lymphoma, to abrogate the therapeutic effect of rituximab [25]. In vitro analysis suggested that this effect of ITZ was specific to lipid-raft-associated molecules, and ITZ impaired alemtuzumab-induced cell death in a dose dependent manner.
Human data
In addition to the extensive pre-clinical data outlined above, there has also been a range of clinical studies performed to assess the therapeutic effects of ITZ in cancer. Note that these are studies looking specifically at the anti-cancer effect of ITZ; studies and trials which have assessed the impact of ITZ, as a CYP 3A4 inhibitor, on the pharmacokinetics of other anticancer agents have not been included. Also not included are a number of studies of ITZ as an anti-fungal prophylactic in cancer patients undergoing treatment.
Based on the findings that ITZ could potentially reverse resistance to daunorubicin in a murine leukaemia cell line [14], Vreugdenhil and colleagues analysed data from a double-blinded randomised clinical trial of ITZ as an anti-fungal prophylactic in neutropenic leukaemia patients treated with daunorubicin [26]. The analysis included 23 patients with acute lymphoblastic leukaemia (ALL), of whom 11 received ITZ, and 42 patients with acute myeloid leukaemia (AML), of whom 17 received ITZ. Results showed that in ALL patients, disease-free survival (DFS) tended to be longer in the ITZ group as compared to control (P < 0.06), but the remission rate was not significantly different. In the AML patients, DFS and remission rates were similar in the ITZ and control groups.
A phase II non-comparative randomised study investigated two dose schedules of ITZ monotherapy in men with castration-resistant metastatic prostate cancer [27]. The low dose (200 mg/day) arm closed early after accruing 17 patients because of a pre-specified futility rule. The high dose arm (600 mg/day) completed, and accrued 29 patients. The primary endpoint was the prostate-specific antigen (PSA) progression-free survival (PFS) rate at 24 weeks, with a 45% success rate required to achieve statistical significance. Results showed that at 24 weeks the PFS rate was 11.8% for the low dose arm, and 48% for the high dose arm. Median PFS, a secondary endpoint, was 11.9 weeks and 35.9 weeks in the two arms respectively. The PFS value of 35.9 weeks is of a range similar to other investigational drugs in this patient population. One patient in the low dose arm and two patients in the high dose arm experienced partial response according to response evaluation criteria in solid tumors (RECIST) criteria. Of note, ITZ treatment also had positive effects on circulating tumour cell counts and Hedgehog signalling was shown to be reduced in skin biopsy samples. No changes were reported for plasma vascular endothelial growth factor (VEGF) levels in either arm. Toxicity was greater in the high dose arm, with the most common side effects being fatigue, nausea, anorexia, rash, and a syndrome of hypokalaemia, hypertension, and oedema. Although there were no grade 4 toxicities, 4 (14%) patients in the high dose arm came off study because of toxicity (one not drug related). There was no negative impact on testosterone levels in either treatment arm.
A case report has also been published by the same group [28]. A 65-year old patient with biochemically recurrent prostate cancer unwilling to undergo castrating treatment was treated with high dose ITZ (300 mg b.i.d.). Treatment with ITZ caused a >50% reduction of PSA level after 12 weeks with no significant change in testosterone level. However, treatment was stopped after five months because of elevated bilirubin levels (which returned to normal range on treatment cessation). The 50% decrease in PSA levels was maintained during ITZ treatment but increased after ITZ discontinuation.
The pre-clinical evidence of anti-angiogenic activity in NSCLC was followed by a phase II trial as a second line therapy in metastatic nonsquamous NSCLC [29]. Patients were randomised to pemetrexed(PM) with or without ITZ (PM ITZ) at a dose of 200 mg/day, on a 21-day cycle, with PM 500 mg/m2 on day 1, and ITZ daily. Treatment was intended to last until disease progression, although the trial had to complete early because of the increased usage of PM in a first-line setting. The intended accrual had been 112 patients, and primary end points included the progression-free survival rate at three months. The number of patients actually enrolled was 23, with 15 randomised to PM ITZ, and 8 to PM alone. At three months, the PFS rate for PM ITZ was 67%, versus 29% on the PM arm (P = 0.11). Median PFS was 5.5 months for PM ITZ compared to 2.8 months for PM (hazard ratio = 0.399, P = 0.089). Median overall survival (OS) for patients receiving PM ITZ was 32 months versus eight months for PM (hazard ratio = 0.194, P = 0.012). Toxicities were equivalent in the two arms. While the small sample size makes it difficult to draw firm conclusions from the results, the authors report an intention to launch a future phase II trial in a more relevant clinical context–cisplatin and gemcitabine with and without ITZ, with additional end-points including assessments of tumour blood flow and hypoxia.
Results have also been reported for a small phase II trial in basal cell carcinoma (BCC) [30]. In this small open label trial, two cohorts of patients were treated either with 200 mg b.i.d. for one month (15 patients), or 100 mg b.i.d. for an average of 2.3 months (four patients). Primary end points were changes in proliferative and Hedgehog-related biomarkers. Secondary end points included change in tumour size for a subset of patients with multiple non-biopsied tumours. Results showed a reduction in tumour cell proliferation (as measured by Ki67 staining) of 45% (P = 0.04), Hedgehog pathway activity (as measured by GLI1 mRNA) of 65% (P = 0.03), and reduced tumour area of 24% (95% CI, 18.2–30.0%). In a subset of patients previously treated with the Hedgehog-inhibitor vismodegib, and in control patients, there were no changes in cell proliferation or tumour size. Of eight patients with multiple non-biopsied tumours, four achieved partial response, and four had stable disease. Toxicity was low: one patient withdrew because of grade 2 fatigue, and one patient withdrew because of congestive heart failure from previous chemotherapy with Adriamycin.
A retrospective analysis of patients with recurrent ovarian clear cell carcinoma treated with a combination of chemotherapy and adjunctive ITZ was performed by Inoue and colleagues [31]. Patients who had progressed during the first-line treatment or during platinum-based chemotherapy at recurrence and who continued chemotherapy were included. ITZ was used with the aim of potentiating the action of the chemotherapy drugs and was selected because of the preclinical evidence for ITZ inhibition of drug efflux and angiogenesis. Eight patients were identified who had received docetaxel and carboplatin with ITZ, (oral solution at a dose of 400 mg/day, days -2 to 2 on a two-week cycle). The response rate was 44%, median PFS was 544 days (95% CI = 82–544 days) and median OS was 1047 days (95% CI = 462–1332 days). The authors deemed the figures encouraging in a patient population with disease that rarely responds to chemotherapy. A subsequent analysis, also retrospective, looked at refractory ovarian cancer patients treated with adjunctive ITZ as part of second or later chemotherapy regimen [32]. Of 55 patients with refractory ovarian cancer, 18 received ITZ as part of a second or third line protocol. The ITZ dose was 400–600 mg, oral solution administered on days -2 to 2 or 3, on a two-week cycle. Patients not treated with ITZ received a range of second or later chemotherapies, including pegylated liposomal doxorubicin, gemcitabine, docetaxel, and other standard drugs. Overall, the chemotherapy response rate was 18% (10 of 55 patients), however, the response rate for ITZ was 32% (six patients of 19), whereas it was only 11% (four of 36) for those not receiving ITZ, (P = 0.06). In terms of PFS, median PFS for ITZ was 103 days (95% CI > 84 days) and 53 days for non-ITZ (95% CI = 38–88 days), (P = 0.014). Median OS for ITZ was 642 days (95% CI = 238–1166 days) and for non-ITZ it was 139 days (95% CI = 89–183 days), (P = 0.006). The ITZ hazard ratio was 0.24 (95% CI = 0.10–0.60; P = 0.002) for PFS and 0.27 (95% CI = 0.11–0.68; P = 0.006) for OS.
The same team also performed a retrospective analysis of heavily pre-treated triple negative breast cancer patients with recurrent disease [33].Thirteen patients were identified who had been treated with two or more lines of chemotherapy, 12 of whom had metastatic disease in the lungs, liver, or brain. All patients were subsequently treated with a regimen of docetaxel, carboplatin, and gemcitabine with adjunctive ITZ on a two week cycle. The ITZ dose used was 400 mg, administered on days −2 to 2 of the chemotherapy treatment. The response rate was 65% (95% CI = 35–88%). The median PFS was 10.8 months (95% CI, 7.6–15.3 months), and the median OS was 20.4 months (95% CI: 13.1–41.4 months), with data on three patients censored. Again, despite the low number of patients and the retrospective nature of the analysis, the authors deemed these results encouraging.
A pilot trial of ITZ pharmacokinetics in patients with metastatic breast cancer has also reported results. Patients (n = 14) received oral ITZ at a dose of 200 mg/day until disease progression or unacceptable toxicity. Primary outcomes were pharmacokinetic data, (plasma levels of ITZ and hydroxyitraconazole), correlated with measures of angiogenesis—plasma VEGF-A and thrombospondin -1 (TSP-1), serum basic fibroblast growth factor (bFGF), and placental growth factor PlGF—at baseline, two and four weeks. Median ITZ/hydroxyitraconazole levels at two and four weeks were 181/331.5 ng/mL and 202/337 ng/mL respectively. Median bFGF and PlGF levels decreased with administration of ITZ from baseline to weeks two and four. The bFGF displayed high correlations with ITZ and hydroxyitraconazole at weeks two and four although not statistically significant. Plasma TSP-1 increased at weeks two and four. VEGF-A levels increased from baseline to week two, but decreased with drug administration during weeks two to four. PlGF, TSP-1, VEGF-A did not correlate with drug levels. Of 13 evaluable patients, one had a partial resonse (PR), three stable disease (SD), and nine progressive disease (PD). Estimated time to progression and OS were 1.8 and 19.3 months respectively [34].
A report has also been published detailing a case of pancreatic cancer which showed response to ITZ treatment [35]. The patient was a heavily pre-treated 64-year- old male with unresectable stage III pancreatic adenocarcinoma who developed disseminated histoplasmosis following his third cycle of gemcitabine. He was treated with ITZ for nine months, without concurrent chemotherapy or other treatment, at which point his pancreatic cancer was reassessed and found to be resectable. Following successful surgical resection, the patient was followed for a period of four years and showed no signs of recurrence or metastatic disease. However, he later reported weight-loss and ill-health and a scan revealed a new primary cancer, shown to be NSCLC. The treating physicians assessed that the reduction in pancreatic tumour had been caused by the ITZ treatment.
Additionally, a case report exists of a patient suffering from mycosis fungoides, a common form of cutaneous T-cell lymphoma [36]. The patient, a 55-year old man, was treated with ITZ at 200 mg/day for seven days in case his symptoms were due to seborrhoeic dermatitis. Symptomatic improvement was noted within 3 days and all lesions had disappeared by the end of treatment. A subsequent recurrence was similarly treated with ITZ and again showed a response. The diagnosis of mycosis fungoides was made on the basis of histological features from repeated biopsies and lack of evidence of any fungal infection and the response to ITZ was therefore unexpected.
Clinical trials
Data for clinical trials as assessed on 23rd February 2015.
NCT01787331—A phase II study of ITZ in biochemical relapse in prostate cancer. This single-arm trial is currently recruiting. Patients with non-castrate, non-metastatic, biochemically relapsed prostate cancer who have received prior definitive local therapy are prescribed oral ITZ at a dose of 600 mg/day (300 mg b.i.d.). The primary outcome measure is the proportion of men who experience a > 50% reduction of PSA level after 12 weeks of treatment. There are numerous secondary objectives including time to PSA progression, median metastasis free survival (MFS), and a number of analyses of Hedgehog pathway response, and clinical response.
NCT02357836—A phase 0 study of neo-adjuvant use of ITZ in NSCLC prior to surgical resection. Following base-line assessment of angiogenic and Hedgehog pathway activity patients will receive seven to ten days of oral ITZ at a dose of 600 mg per day, and then be reassessed. This is a pharmacodynamics study looking for evidence of anti-angiogenic and Hedgehog pathway inhibition, in addition to assessing patient’s safety.
NCT02120677—Topical ITZ in the treatment of basal cell carcinoma. This single arm safety study is currently recruiting. Patients are treated with a topical solution of 50% ITZ compounded in petrolatum jelly for between three to seven days. The primary outcome is a measure of Hedgehog (Gli) response. Secondary measures are related to toxicity.
NCT02354261—Basal cell carcinoma nevus syndrome (BCCNS) is a familial cancer pre-disposition syndrome associated with mutations in the Hedgehog signalling pathway. Affected individuals are prone to development of BCC and other cancers. This open-label phase II trial will use a new formulation of ITZ called SUBA-itraconazole at a daily dose of 200 mg (100 mg b.i.d.) in BCCNS patients. The primary outcome is disease response rate. Secondary outcomes are safety and tolerability measures, the duration of responses, and the number of new lesions susceptible to surgical resection.
Additionally, a team at Stanford University is planning to assess the combination of ATO and ITZ in BCC. A current trial, NCT01791894, is investigating the use of ATO. A new trial with the combination is planned for 2015/2016 (Jean Tang, personal communication).
NCT02366884—This single-blinded two-centre phase II trial will utilise a range of anti-bacterial, anti-fungal (including ITZ) and anti-protozoal agents in combinations against all cancer types. The trial is testing a theory that cancer is ‘atavistic’ and shares many of the pathways and mechanisms used by unicellular organisms and that inhibition of these pathways using existing medications for these indications may inhibit cancer. Patient recruitment is aimed at those with terminal disease and no standard of care options. The primary outcome is objective clinical tumour regression rate.
Itraconazole is also a component of the CUSP9* protocol for glioblastoma [37]. This is a multi-agent cocktail of repurposed drugs to be used in addition to standard of care for recurrent glioblastoma. A clinical trial of CUSP9* is currently planned for 2015 (Marc-Eric Halatsch, personal communication).
Mechanism of action
There are multiple mechanisms of action proposed to explain the diverse anticancer effects of ITZ. These include:
• Anti-angiogenic
• Hedgehog pathway inhibition
• Autophagy induction
• Reversal of multi-drug resistance
Angiogenesis
A screen by Chong et al of FDA approved drugs using a human endothelial cell proliferation assay indicated that ITZ is a potent inhibitor of endothelial cell proliferation at easily achievable plasma levels, with little or no effect on non-endothelial cells [17]. In contrast to other members of the azole anti-fungals (ketoconazole, fluconazole, and voriconazole), ITZ inhibited proliferation, with an IC50 of 0.16 μM. The mechanism appeared to be related to cell cycle arrest at the G1 phase. In vivo in a Matrigel mouse model, ITZ administered intraperitoneally with a dose equivalent to human IV dosing, showed a 67.5% decrease of new blood vessel formation compared to untreated controls. The anti-fungal activity of ITZ is because of the inhibition of lanosterol 14α-demethylase (14DM), required to preserve membrane integrity in fungal cells. In humans 14DM is involved in the biosynthesis of cholesterol. In vitro the anti-angiogenic effect of ITZ was reduced in the presence of cholesterol. Subsequent analysis has shown that ITZ also caused the inhibition of mammalian target of rapamycin (mTOR) activity and proper cholesterol trafficking in endothelial cells [38].
Rudin and colleagues further investigated the anti-angiogenic activity of ITZ in xenograft models of NSCLC [19]. Immunocompromised mice were injected with LX-14 (squamous cell) and LX-7 (adenocarcinoma) cells derived from primary tumour samples from untreated NSCLC patients. In vitro analysis with HUVECs indicated that ITZ inhibited cell migration, chemotaxis, and tube formation. The mouse models were treated with ITZ orally at a dose of 75 mg/kg and both models showed significant reductions in tumour volume. Over a 14 day period ITZ monotherapy in LX-14 and LX-7 mice resulted in 72% and 79% inhibition of tumour growth, respectively, compared to vehicle treated controls (P < 0.001). Combination therapy with cisplatin was superior to cisplatin monotherapy to a statistically significant extent (P ≤ 0.001 compared to ITZ or cisplatin alone) resulting in over 95% growth inhibition but no tumour regression. Treatment with ITZ also decreased tumour vascular area but increased expression of HIF1α.
Further insight into the anti-angiogenic mechanisms of action were provided by investigations into the effects of ITZ on VEGF signalling, specifically showing that ITZ interferes with VEGF binding to VEGFR2 [39]. However, in the metastatic breast cancer trial expression of pro-angiogenic factors (VEGF-A and PlGF) were reduced and levels of the anti-angiogenic factor TSP-1 increased, this was not correlated with drug or metabolite levels [34]. Furthermore the trial in prostate cancer showed no evidence of a change in plasma VEGF levels with ITZ at 200 mg/day and 600 mg/day [27].
However, indirect clinical evidence of anti-angiogenic activity is provided by a case series report in infantile hemangiomas [40]. Physicians treating a case of infantile hemangioma with concurrent fungal infection found that treatment with a range of drugs, including ITZ, resolved the fungal infection and also caused regression of the hemangioma. Subsequent ITZ treatment in five other infants, including four without evidence of fungal infection, also produced regression of lesions.
Hedgehog inhibition
The Hedgehog pathway is activated in a number of cancer types and is felt to play a major role in the maintenance of cancer stem cells, a relatively chemo- and radio-resistant subset of cancer cells [41]. Thus the notion of treating cancer with agents that target both stem and non-stem cell populations has gained momentum, and ITZ, as a Hedgehog inhibitor, may well play a pivotal role in such therapies. Indeed one indication that ITZ may have such a role comes from in vivo work in a multiple myeloma (MM) model in which ITZ increased survival in mice injected with MM stem cells [42].
As noted earlier, initial identification of ITZ as a Hedgehog pathway inhibitor came about via a screen of Food and Drug Administration (FDA) approved drugs [20]. While many drugs were identified as potential Hedgehog pathway inhibitors, few candidates did so at clinically achievable concentrations. In contrast ITZ was shown to have an IC50 of 800 nM, a value approximately ten-fold lower than that of ketoconazole, which had an IC50 of 9 μM. The primary metabolite, hydroxyitraconazole, also inhibited the Hedgehog pathway, with an IC50 of approximately 1.2 μM. Other azole anti-fungals, for example fluconazole, did not show evidence of anti-Hedgehog activity, suggesting that the mechanism of action is not directly related to the anti-fungal activity of this class of drugs. Mechanistic analysis indicated that the anti-Hedgehog activity is via Smoothened, and that it acts in a manner distinct from cyclopamine and other Smoothened antagonists. Later work has investigated the use of ITZ in tumours with acquired resistance to a range of Smoothened inhibitors in clinical development [22].
The finding that ITZ inhibits Hedgehog signalling was confirmed using skin biopsy samples in the phase II randomised trial in men with castration-resistant prostate cancer [27]. A secondary outcome of the trial was an analysis of Hedgehog pathway activity in skin punch biopsies at baseline and at four and 12 weeks after commencement of treatment. GLI1 mRNA expression was used as a marker for Hedgehog pathway activation. Results showed that GLI1mRNA expression was reduced in 33% and 68% of patients in the low- and high-dose arms of the trial. The percentage of patients who showed greater than 50% reduction was 28%, in contrast to 68% in studies using the Hedgehog inhibitor vismodegib. In terms of clinical results, there was a significant correlation between longer PSA PFS and GLI1 reduction (P = 0.028), and a trend towards longer PFS (P = 0.128).
Autophagy
ITZ was found to induce autophagic cell growth arrest in U87 and C6 glioblastoma cells both in vitro and in a mouse xenograft (U87) model [24]. The effect is related to inhibition of mTOR signalling, which is caused by the blockade of cholesterol trafficking by ITZ, as also noted in endothelial cells [38]. ITZ also inhibited AKT1, an upstream regulator of mTOR, and that reactivation of AKT1 reversed the induction of autophagy and growth arrest. Inhibition of autophagy abrogated the reduction of cellular proliferation, suggesting that the use of ITZ with autophagy inhibitors may be problematic.
Drug resistance
Acquired or multi-drug resistance (MDR) is a common phenomenon in medical treatment, with multiple mechanisms of action including the activity of the drug efflux proteins of the ATP binding cassette (ABC) transporter family. The most well-known of these is P-glycoprotein (P-gp), which is known to be associated with resistance to a wide range of therapeutic agents, including antibiotics and cytotoxic chemotherapy drugs. Reversal of MDR as a clinical strategy to improve response to treatment has been actively investigated for some years [43]. ITZ, in common with some related compounds such as ketoconazole, has been shown to be a potent inhibitor of P-gp at clinically relevant doses [44]. In a cell line over-expressing P-gp, ITZ was able to reduce P-gp transporter function by 50% at a dose of approximately 2 μM.
Another member of the ABC transporter family is human breast cancer resistance protein (BCRP), which is implicated in resistance to a range of active anticancer agents, including anthracyclines, methotrexate, topotecan, mitoxantrone, and other drugs [45]. In an in vitro study, HEK cells over-expressing BCRP and resistant to topotecan were treated with a range of azole anti-fungals and tested for efflux of a fluorescent marker [16]. Treatment with ITZ at a dose of 0.1 and 1.0 μM increased intracellular fluorescence by 20% and 40% respectively, showing inhibition of BCRP. ITZ at doses of 2 μM and 5 μM also significantly reversed resistance to the cytotoxic effect of topotecan.
Our take
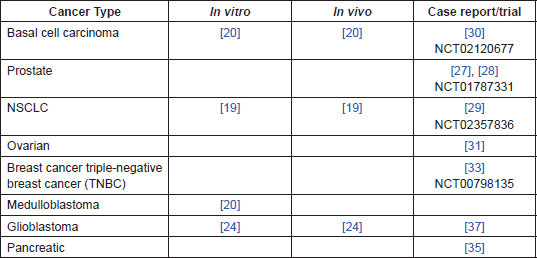
The evidence presented above, (and summarised in Table 1), suggests that ITZ has a number of anti-cancer effects at clinically achievable doses, particularly in NSCLC, BCC, and prostate cancer. There is evidence that that it may also be applicable to a number of other cancers, including glioblastoma, breast, pancreatic, and ovarian. More generally, the main mechanisms of action investigated to date, antiangiogenic and Hedgehog inhibition, may serve to identify other cancer types where investigation with ITZ may be beneficial.
We note that the experience with many targeted therapies suggest that resistance may become an issue with ITZ as a monotherapy, as it is, for example with another Hedgehog inhibitor, vismodegib [46]. Given the evidence that ITZ acts on a different molecular target to vismodegib, the combination of the two drugs may act to limit the acquisition of such resistance. The combination warrants investigation in a clinical trial with some degree of urgency as vismodegib progresses into clinical use for advanced BCC and other malignancies. There is also intriguing evidence that Hedgehog inhibitors may act to improve immune surveillance and adaptive immune response in BCC, with a suggestion that this may be exploited clinically with the addition of other immunostimulant therapies [47]. Confirmation of this finding in ITZ would add a significant additional mechanism of action with possible clinical benefit and as such further investigation is warranted.
As an ‘old’ drug, it may be that ITZ has multiple molecular targets such that acquired resistance is less of an issue than with agents such as vismodegib which are closely targeted to a single pathway. It would be interesting to combine ITZ with a range of other drugs, including, but not limited to, cytotoxic chemotherapies. The pre-clinical and clinical data with platinum-based chemotherapies and the clinical data with pemetrexed are quite promising. Conceptually, combinations with drugs that target other points in the Hedgehog pathway, or with drugs that target other mechanisms of stem cell survival, or with drugs targeting non-stem cells, all make eminent sense. A number of possible such combinations are outlined in the supplementary materials.
Table 1. Summary of evidence by cancer type.
In addition to showing evidence of clinical activity at standard ‘on-label’ therapeutic doses, ITZ can also be used for long periods with generally manageable levels of toxicity. This is particularly important in the context of metronomic chemotherapy schedules, and it may well be that ITZ can serve as a useful adjunct to such protocols. New formulations of ITZ are also being developed commercially which offer greater bioavailability than the standard generic compound. One example is SUBA-Itraconazole (licensed as Lozanoc), developed by Mayne Pharmaceuticals, and being investigated as a possible anti-cancer agent by HedgePath Pharmaceuticals in the trial for BCCNS.
Next steps
The evidence is strongest for clinical trials of ITZ, in combination with other agents, in the following cancer types:
• NSCLC
• BCC
• Prostate cancer
• Glioblastoma
• Ovarian carcinoma
• Metastatic breast cancer
• Pancreatic cancer
Clinical trials in some of these are on-going, but in others the potential of ITZ is yet to be explored. In particular it should be noted that ITZ could also be of interest in other cancers in which the Hedgehog pathway is known to play an important role. These include rare cancers such as SCLC, medulloblastoma, or certain types of sarcomas (rhabdomyosarcoma, chondrosarcoma, and osteosarcoma), for which very few new drugs are currently being developed, making it an interesting candidate for rapid implementation of clinical trials in these diseases with high unmet needs.
Conclusion
The evidence for an anti-cancer effect of ITZ treatment comes from in vitro, in vivo, and human data. It has well-established pharmacokinetics and a known toxicity profile making this generic drug a strong candidate for repurposing as an oncological treatment, both in combination with existing standard of care treatments and with other repurposed drugs. A number of possible multi-drug combinations are outlined, along with their rationale, in the hope that clinicians can initiate clinical trials as a matter of some urgency.
Author contributions
Primary author: Pan Pantziarka. Contributing authors: Vidula Sukhatme, Gauthier Bouche, Lydie Meheus, Vikas P Sukhatme. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests. All the authors are associated with not for profit organisations that aim to repurpose drugs for oncology treatments.
References
1. Lestner J and Hope WW (2013) Itraconazole: an update on pharmacology and clinical use for treatment of invasive and allergic fungal infections Expert opinion on drug metabolism & toxicology 9(7) pp 911–26
2. Joint_Formulary_Committee (2015) British National Formulary 69th ed., BMJ Group and Pharmaceutical Press
3. Gupta AK, Cooper EA and Ginter G (2003) Efficacy and safety of itraconazole use in children Dermatologic Clinics 21(3) pp 521–535
4. Barone JA et al (1993) Food interaction and steady-state pharmacokinetics of itraconazole oral solution in healthy volunteers Pharmacotherapy 18(4) pp 295–301
5. Barone JA et al (1998) Enhanced bioavailability of itraconazole in hydroxypropyl-beta- cyclodextrin solution versus capsules in healthy volunteers Antimicrob Agents Chemother 42(7) pp 1862–5 PMID: 9661037 PMCID: 105699
6. Poirier JM and Cheymol G (1998) Optimisation of itraconazole therapy using target drug concentrations Clin Pharmacokinet 35(6) pp 461–73 DOI: 10.2165/00003088-199835060-00004
7. Felton T, Troke PF and Hope WW (2014) Tissue penetration of antifungal agents Clin Microbiol Rev 27(1) pp 68–88 DOI: 10.1128/CMR.00046-13 PMID: 24396137 PMCID: 3910906
8. Odds FC and Bossche HV (2000) Antifungal activity of itraconazole compared with hydroxy-itraconazole in vitro J Antimicrob Chemother 45(3) pp 371–3 DOI: 10.1093/jac/45.3.371 PMID: 10702560
9. Mc Evoy GK and Snow EK (2008) AHFS: Drug Information Bethesda MD American Society of Health-System Pharmacists
10. Bermúdez M et al (2005) Itraconazole-related increased vincristine neurotoxicity: case report and review of literature J Pediatr Hematol Oncol 27(7) 389–92 DOI: 10.1097/01.mph.0000172751.06286.5b PMID: 16012330
11. Takahashi N et al (2008) Itraconazole oral solution enhanced vincristine neurotoxicity in five patients with malignant lymphoma Intern Med 47(7) 651–3 DOI: 10.2169/internalmedicine.47.0701 PMID: 18379154
12. Summers KK et al (1997) Therapeutic drug monitoring of systemic antifungal therapy J Antimicrob Chemother 40(6) pp 753–64 DOI: 10.1093/jac/40.6.753
13. Kim JS et al (2014) The relationship between the success rate of empirical antifungal therapy with intravenous itraconazole and clinical parameters, including plasma levels of itraconazole, in immunocompromised patients receiving itraconazole oral solution as prophylaxis: a Ann Hematol 93(1) pp 33–42 DOI: 10.1007/s00277-013-1826-x
14. Gupta S, Kim J and Gollapudi S (1991) Reversal of daunorubicin resistance in P388/ADR cells by itraconazole J Clin Invest 87(4) pp 1467–9 DOI: 10.1172/JCI115154 PMID: 1849151 PMCID: 295200
15. Kurosawa M et al (1996) Reversal effect of itraconazole on adriamycin and etoposide resistance in human leukemia cells Ann Hematol 72(1) pp 17–21 DOI: 10.1007/BF00663011 PMID: 8605275
16. Gupta A, Unadkat JD and Mao Q (2007) Interactions of azole antifungal agents with the human breast cancer resistance protein (BCRP) J Pharm Sci 96(12) pp 3226–35 DOI: 10.1002/jps.20963 PMID: 17518356
17. Chong CR et al (2007) Inhibition of angiogenesis by the antifungal drug itraconazole ACS Chem Biol 2(4) pp 263–70 DOI: 10.1021/cb600362d PMID: 17432820
18. Nacev BA and Liu JO (2011) Synergistic inhibition of endothelial cell proliferation, tube formation, and sprouting by cyclosporin A and itraconazole PloS One 6(9) p e24793 DOI: 10.1371/journal.pone.0024793 PMID: 21969860 PMCID: 3182171
19. Aftab BT et al (2011) Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer Cancer Res 71(21) pp 6764–72 DOI: 10.1158/0008-5472.CAN-11-0691 PMID: 21896639 PMCID: 3206167
20. Kim J et al (2010) Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth Cancer Cell 17(4) pp 388–99 DOI: 10.1016/j.ccr.2010.02.027 PMID: 20385363 PMCID: 4039177
21. Sánchez C et al (1995) Treatment of cerebral Aspergillosis with itraconazole: do high doses improve the prognosis? Clin Infect Dis 21(6) pp 1485–7 DOI: 10.1093/clinids/21.6.1485 PMID: 8749640
22. Kim J et al (2013) Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists Cancer Cell 23(1) pp 23–34 DOI: 10.1016/j.ccr.2012.11.017 PMID: 23291299 PMCID: 3548977
23. You M et al (2014) Targeting of the Hedgehog signal transduction pathway suppresses survival of malignant pleural mesothelioma cells in vitro J Thorac Cardiovasc Surg 147(1) pp 508–16 DOI: 10.1016/j.jtcvs.2013.08.035
24. Liu R et al (2014) Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking Autophagy 10(7) pp 1241–55 DOI: 10.4161/auto.28912 PMID: 24905460 PMCID: 4203550
25. Ringshausen I et al (2010) Antifungal therapy with itraconazole impairs the anti-lymphoma effects of rituximab by inhibiting recruitment of CD20 to cell surface lipid rafts Cancer Res 70(11) pp 4292–6 DOI: 10.1158/0008-5472.CAN-10-0259 PMID: 20460536
26. Vreugdenhil G et al (1993) Itraconazole and multidrug resistance: possible effects on remission rate and disease-free survival in acute leukemia Ann Hematol 67(3) pp 107–9 DOI: 10.1007/BF01701730 PMID: 8396989
27. Antonarakis ES et al (2013) Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer Oncologist 18(2) pp 163–73 DOI: 10.1634/theoncologist.2012-314 PMID: 23340005 PMCID: 3579600
28. Suzman DL and Antonarakis ES (2014) High-dose itraconazole as a noncastrating therapy for a patient with biochemically recurrent prostate cancer Clin Genitourin Cancer 12(2) pp e51–3 DOI: 10.1016/j.clgc.2013.11.015
29. Rudin CM et al (2013) Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer J Thorac Oncol 8(5) pp 619–23 PMID: 23546045 PMCID: 3636564
30. Kim DJ et al (2014) Open-Label, Exploratory Phase II Trial of Oral Itraconazole for the Treatment of Basal Cell Carcinoma J Clinic Oncol 32(8) pp 745–51 DOI: 10.1200/JCO.2013.49.9525
31. Tsubamoto H et al (2014) Impact of combination chemotherapy with itraconazole on survival for patients with recurrent or persistent ovarian clear cell carcinoma Anticancer Res 34(4) pp 2007–14 PMID: 24692739
32. Tsubamoto H et al (2014) Impact of combination chemotherapy with itraconazole on survival of patients with refractory ovarian cancer Anticancer Res 34(5) pp 2481–7 PMID: 24778064
33. Tsubamoto H, Sonoda T and Inoue K (2014) Impact of Itraconazole on the Survival of Heavily Pre-treated Patients with Triple-Negative Breast Cancer Anticancer Res 34(7) pp 3839–44 PMID: 24982411
34. Ademuyiwa FO et al (2011) A pilot trial of itraconazole pharmacokinetics in patients with metastatic breast cancer J Clin Oncol suppl pp abstr e13565
35. Lockhart NR, Waddell JA and Schrock NE (2015) Itraconazole therapy in a pancreatic adenocarcinoma patient: A case report J Oncol Pharm Pract DOI: 10.1177/1078155215572931 PMID: 25670260
36. Cooper SM, Sheridan A and Burge S (2003) Mycosis fungoides responding to systemic itraconazole Journal of the European Academy of Dermatology and Venereology 17 pp 588–590
37. Kast RE, Karpel-Massler G and Halatsch M (2014) CUSP9* treatment protocol for recurrent glioblastoma: aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide Oncotarget 5(18) pp 8052–82 PMID: 25211298 PMCID: 4226667
38. Xu J et al (2010) Cholesterol trafficking is required for mTOR activation in endothelial cells Proc Nat Acad Sci USA 107(10) pp 4764–9 DOI: 10.1073/pnas.0910872107 PMID: 20176935 PMCID: 2842052
39. Nacev BA et al (2011) The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells J Biol Chem 286(51) pp 44045–56 DOI: 10.1074/jbc.M111.278754 PMID: 22025615 PMCID: 3243534
40. Ran Y et al (2015) Successful treatment of oral itraconazole for infantile hemangiomas: A case series J Dermatol (February) 42(2) pp 202–6 DOI: 10.1111/1346-8138.12724
41. Yu Z et al (2012) Cancer stem cells Int J Biochem Cell Biol 44(12) pp 2144–2151 DOI: 10.1016/j.biocel.2012.08.022 PMID: 22981632 PMCID: 3496019
42. Yang Y et al (2013) RARα2 expression confers myeloma stem cell features Blood 122(8) pp 1437–47 DOI: 10.1182/blood-2013-02-482919 PMID: 23847194 PMCID: 3750340
43. Callaghan R, Luk F and Bebawy M (2014) Inhibition of the multidrug resistance P-glycoprotein: time for a change of strategy? Drug Metab Dispos 42(4) pp 623–31 DOI: 10.1124/dmd.113.056176 PMID: 24492893 PMCID: 3965902
44. Wang E et al (2002) Interaction of common azole antifungals with P glycoprotein Antimicrob Agents Chemother 46(1) pp 160–5 DOI: 10.1128/AAC.46.1.160-165.2002
45. Noguchi K, Katayama K and Sugimoto Y (2014) Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics Pharmacogenomics Pers Med 7 pp 53–64 DOI: 10.2147/PGPM.S38295
46. Pricl S et al (2014) Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma Mol Oncol 9(2) 389–97 DOI: 10.1016/j.molonc.2014.09.003 PMID: 25306392
47. Otsuka A et al (2015) Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma Clin Cancer Res 21(6) 1289–97 DOI: 10.1158/1078-0432.CCR-14-2110 PMID: 25593302
Repurposing Drugs in Oncology (ReDO)—itraconazole as an anti-cancer agent–supplementary material
Introduction
The following drugs warrant further investigation in combination with itraconazole (ITZ) and existing standard of care cancer treatments in a range of cancers. These combinations, listed in Table 1, have been selected on the basis of existing pre-clinical and clinical experience in each of the indications. In some cases these combinations replicate existing protocols currently being tested in clinical trials, but substitute known and repurposed drugs for the newer and/or more toxic agents currently being investigated. All of these proposed combinations are expected to display relatively low toxicity and use low cost and generally available agents. The following drugs are not listed in order of priority.
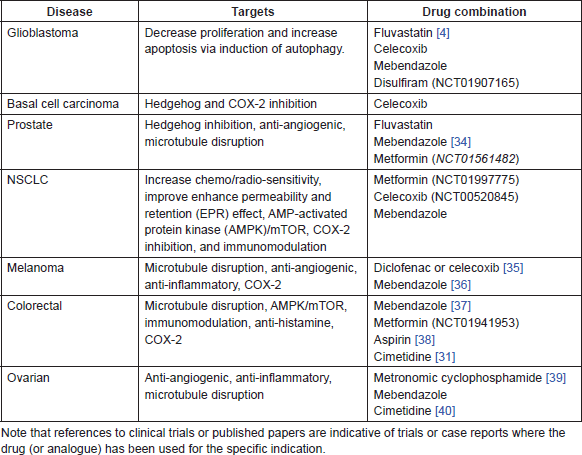
Table 1. Proposed drug combinations with ITZ and standard of care in different cancers.
Cancer stem cells
The following combination therapies are aimed at the elimination of cancer stem cell or tumour-initiating cell populations, in combination with ITZ, via Hedgehog pathway inhibition.
• Fluvastatin—There is both epidemiological and pre-clinical evidence of an anti-cancer effect of lipophilic statins, including simvastatin, lovastatin, and fluvastatin. Multiple mechanisms of action have been proposed and these are summarised in [1]. A number of clinical trials are also currently being carried out investigating the addition of statins to existing standard of care treatments in a wide range of cancers, including prostate, breast, NSCLC, glioblastoma, colorectal, and gastric cancers. There is evidence that concurrent use of ITZ and some statins, such as simvastatin and lovastatin, causes increased toxicity, particularly increased rhabdomyolysis [2, 3]; however, this increased toxicity is not apparent with fluvastatin [2]. Clinical trials of fluvastatin as an anti-cancer agent include for early stage breast cancer, prostate cancer, and optochiasmatic gliomas. Evidence exists that fluvastatin exerts an anti-proliferative effect via induction of autophagy [4, 5]. There is also in vitro evidence that fluvastatin acts directly on stem-like cell populations [4]. The combination with ITZ may exert a synergistic pro-autophagic effect, particularly in glioblastoma and other malignancies where autophagy has an anti-tumour effect.
• Mebendazole—There is a range of pre-clinical and clinical evidence of an anti-cancer effect for the widely used anti-helminthic drug mebendazole. There are a number of distinct possible mechanisms of action and the drug currently being assessed in a number of clinical trials as an anti-cancer agent [6]. Mebendazole is known to act as a microtubule-disrupting agent to achieve its anti-parasitic action, and it is suggested that in part this may also play a role in the anti-cancer activity. However, there is also evidence that mebendazole is anti-angiogenic [7] and possibly act on the Hedgehog pathway [8]. The combination with ITZ, which has complementary anti-angiogenic and anti-Hedgehog properties is appealing and may be of clinical benefit in NSCLC, glioblastoma, medulloblastoma, prostate, and ovarian cancer.
• Disulfiram—Long used as a treatment for alcohol abuse, disulfiram is now undergoing extensive investigation as a possible agent to target cancer stem cell populations [9–11]. Other possible mechanisms of action include direct pro-apoptotic effects [12], anti-MMP activity [13], and reversal of drug resistance [14]. In addition to extensive pre-clinical data, disulfiram is undergoing investigation in a number of clinical trials including in glioblastoma, pancreatic, colorectal, and breast cancer. The combination with ITZ may be especially useful in glioblastoma, for which there is extensive pre-clinical evidence of activity. It should be noted that ITZ and disulfiram are both included in the innovative CUSP9* protocol for recurrent GBM [15].
Cancer stem and non-stem cells
The following combinations with ITZ target both stem and non-stem cell populations within tumours. Since existing evidence indicates that ITZ is synergistic with existing cytotoxic chemotherapy drugs including platinum-based agents and the anti-folate pemetrexed, additional investigation of these combinations is clearly warranted. There are some indications, based on in vitro and in vivo analysis, that Hedgehog signalling is implicated in treatment failure in radiotherapy, and that Hedgehog inhibition may be beneficial in combination with radiotherapy and chemo-radiation protocols [16, 17]. Therefore investigation of ITZ as a potential adjunct to radiotherapy is justified.
• Methotrexate—Early stage positive results have been reported for the combination of ITZ and the anti-folate chemotherapeutic pemetrexed in NSCLC [18]. Methotrexate, a commonly used first generation anti-folate drug, has yet to be explored in combination with ITZ. Methotrexate is used to treat a variety of cancers in both maximum tolerate dose (MTD) and metronomic dosing schedules and is one of the core drugs for the treatment of bone and soft tissue sarcomas. There is also increasing evidence that aberrant Hedgehog signalling exists in sarcomas [19], suggesting that ITZ may have some potential in treatment. Therefore, given the similar mechanism of action to pemetrexed and the existing efficacy of methotrexate in sarcomas, the combination with ITZ deserves further clinical investigation.
Anti-angiogenic/immunological
The following combinations with ITZ seek to exploit anti-angiogenic and/or immune-related activity rather than cytotoxicity.
• Metronomic chemotherapy—In contrast to MTD chemotherapy, there is evidence that low-dose metronomic chemotherapy acts primarily through non-cytotoxic means. A variety of existing chemotherapeutic agents have been successfully used in metronomic protocols, including oral cyclophosphamide, capecitabine, methotrexate, etoposide, vinorelbine, and temozolomide [20]. There is evidence that the combination of metronomic chemotherapy with additional anti-angiogenic agents may be beneficial [21]. ITZ inhibits at least two angiogenic pathways, as evidenced by its ability to block VEGF and FGF-induced angiogenesis [22]. There is also a suggestion that inhibition of drug efflux may reduce the risk of acquired resistance to some metronomic treatments [23]. Therefore, as there is evidence that ITZ is both anti-angiogenic and inhibits P-gp, it warrants clinical study in combination with metronomic chemotherapy schedules.
• Celecoxib—The NSAID celecoxib is undergoing clinical investigation in a number of clinical trials in a range of cancer types, including colorectal, basal cell carcinoma, prostate, NSCLC, and paediatric brain tumours. Positive results of the combination of celecoxib and standard of care treatments have been reported in a range of cancers, including prostate [24], colorectal [25], ovarian [26] and for chemo-prevention in high-risk non-melanoma skin cancer [27]. While there are some concerns regarding the effects of ITZ on the pharmacokinetics of NSAIDs, a study in healthy human volunteers showed no significant changes in celecoxib bioavailability with concurrent ITZ [28]. Based on the evidence for both ITZ and celecoxib, the combination warrants clinical investigation in a wide range of cancer types, including basal cell carcinoma, prostate cancer, glioblastoma, colorectal, and ovarian cancers.
• Cimetidine—The well-known H2RA, cimetidine is primarily used to treat peptic ulcers and heartburn, but has also been shown to have pre-clinical and clinical evidence of anti-cancer activity in different cancers [29, 30]. A number of mechanisms of action have been proposed, including effects on cell adhesion, angiogenesis, and a range of immunomodulatory effects. The strongest clinical evidence shows a positive effect on survival when used peri-operatively for early stage colorectal cancer [31]. These anti-cancer effects may enhance the activity of ITZ, particularly with respect to cimetidine’s immunomodulatory effect. Given the proposed evidence of a positive effect on immune surveillance induced by oral Hedgehog inhibitors, the combination with cimetidine should be investigated in a suitable animal model [32]. Caution must be exercised however, as the suppression of gastric acid induced by cimetidine may interfere with the pharmacokinetics of ITZ. One strategy to ameliorate the issue is to administer the ITZ tablets with an acidic drink [33]. However, additional pre-clinical work in an appropriate animal model may be warranted before moving to a clinical trial.
References
1. Osmak M (2012) Statins and cancer: current and future prospects Cancer Lett 324(1) pp 1–12 DOI: 10.1016/j.canlet.2012.04.011 PMID: 22542807
2. Kivistö KT, Kantola T and Neuvonen PJ (1998) Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin Br J Clin Pharmacol 46(1) pp 49–53 DOI: 10.1046/j.1365-2125.1998.00034.x
3. Neuvonen PJ, Kantola T and Kivistö KT (1998) Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole Clin Pharmacol Ther 63(3) pp 332–41 DOI: 10.1016/S0009-9236(98)90165-5 PMID: 9542477
4. Jiang P et al (2014) In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells Br J Cancer 111(8) pp 1562–71 DOI: 10.1038/bjc.2014.431 PMID: 25093497 PMCID: 4200085
5. Qi X-F et al (2013) Autophagy contributes to apoptosis in A20 and EL4 lymphoma cells treated with fluvastatin Cancer Cell Int 13(1) pp 111 DOI: 10.1186/1475-2867-13-111 PMID: 24209962 PMCID: 3832223
6. Pantziarka P et al (2014) Repurposing Drugs in Oncology (ReDO)-mebendazole as an anti-cancer agent Ecancermedicalscience 8 pp 443 DOI: 10.3332/ecancer.2014.485 PMID: 25075217 PMCID: 4096024
7. Bai R-Y et al (2015) Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis Neuro Oncol 17(4) pp 1–10 DOI: 10.1093/neuonc/nou234
8. Larsen AR et al (2014) Repurposing the antihelmintic mebendazole as a hedgehog inhibitor Mol Cancer Ther 14(1) 3–13 DOI: 10.1158/1535-7163.MCT-14-0755-T PMID: 25376612 PMCID: 4297232
9. Triscott J, Pambid M and Dunn S (2014) Bullseye: Targeting cancer stem cells to improve the treatment of gliomas by repurposing Disulfiram Stem Cells (Dayton, Ohio) pp 1–7
10. Hothi P et al (2012) High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells Oncotarget 3(10) pp 1124–36 PMID: 23165409 PMCID: 3717950
11. Wang Y et al (2014) Blocking the formation of radiation-induced breast cancer stem cells Oncotarget 5(11) pp 3743–55 PMID: 25003837 PMCID: 4116517
12. Cheriyan VT et al (2014) Disulfiram suppresses growth of the malignant pleural mesothelioma cells in part by inducing apoptosis PLoS ONE 9(4) e93711 DOI: 10.1371/journal.pone.0093711 PMID: 24690739 PMCID: 3972204
13. Cho HJ et al (2007) Disulfiram suppresses invasive ability of osteosarcoma cells via the inhibition of MMP-2 and MMP-9 expression J Biochem Mol Biol 40(6) pp 1069–76 DOI: 10.5483/BMBRep.2007.40.6.1069 PMID: 18047805
14. Liu P et al (2013) Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells Br J Cancer 109(7) pp 1876–85 DOI: 10.1038/bjc.2013.534 PMID: 24008666 PMCID: 3790184
15. Kast RE, Karpel-Massler G and Halatsch M (2014) CUSP9* treatment protocol for recurrent glioblastoma: aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide Oncotarget 5(18) pp 8052–82
16. Ma J et al (2013) Sonic Hedgehog Signaling Pathway Supports Cancer Cell Growth during Cancer Radiotherapy PLoS ONE 8(6) e65032 DOI: 10.1371/journal.pone.0065032 PMID: 23762282 PMCID: 3677896
17. Chaudary N et al (2012) Hedgehog pathway signaling in cervical carcinoma and outcome after chemoradiation Cancer 118(12) pp 3105–15 DOI: 10.1002/cncr.26635
18. Rudin CM et al (2013) Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer J Thorac Oncol 8(5) pp 619–23 PMID: 23546045 PMCID: 3636564
19. Kelleher FC et al (2012) Prevailing importance of the hedgehog signaling pathway and the potential for treatment advancement in sarcoma Pharmacol Ther 136 pp 153–68 DOI: 10.1016/j.pharmthera.2012.08.004 PMID: 22906929
20. André N, Carré M and Pasquier E (2014) Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol 11(7) 413–31 DOI: 10.1038/nrclinonc.2014.89
21. Digklia A and Voutsadakis IA (2014) Combinations of vascular endothelial growth factor pathway inhibitors with metronomic chemotherapy: Rational and current status World J Exp Med 4(4) pp 58–67 DOI: 10.5493/wjem.v4.i4.58
22. Aftab BT et al (2011) Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer Cancer Res 71(21) pp 6764–72 DOI: 10.1158/0008-5472.CAN-11-0691 PMID: 21896639 PMCID: 3206167
23. Akiyama K et al (2014) Inhibition of Multidrug Transporter in Tumor Endothelial Cells Enhances Antiangiogenic Effects of Low-Dose Metronomic Paclitaxel American J Pathol 185(2) 572–80 DOI: 10.1016/j.ajpath.2014.10.017
24. Sooriakumaran P et al (2009) A randomized controlled trial investigating the effects of celecoxib in patients with localized prostate cancer Anticancer Res 29(5) pp 1483–8 PMID: 19443354
25. Jin C-H et al (2011) Observation of curative efficacy and prognosis following combination chemotherapy with celecoxib in the treatment of advanced colorectal cancer J Int Med Res 39(6) pp 2129–40 DOI: 10.1177/147323001103900609
26. Legge F et al (2011) Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients BMC Cancer 11 pp 214 DOI: 10.1186/1471-2407-11-214 PMID: 21627839 PMCID: 3123659
27. Elmets CA et al (2010) Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial J Natl Cancer Inst 102(24) pp 1835–44 DOI: 10.1093/jnci/djq442 PMID: 21115882 PMCID: 3001966
28. Jayasagar G et al (2003) Effect of itraconazole on the pharmacokinetics of celecoxib in healthy human volunteers Pharmazie 58(11) pp 840–1 PMID: 14664345
29. Pantziarka P et al (2014) Repurposing drugs in oncology (ReDO)-cimetidine as an anti-cancer agent Ecancermedicalscience 8 pp 485 DOI: 10.3332/ecancer.2014.485 PMID: 25525463 PMCID: 4268104
30. Kubecova M et al (2011) Cimetidine: an anticancer drug? Euro J Pharm 42(5) pp 439–44
31. Deva S and Jameson M (2012) Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer The Cochrane Database Syst Rev 8(8) pp CD007814
32. Otsuka A et al (2015) Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma Clin Cancer Res 21(6)1289–97 DOI: 10.1158/1078-0432.CCR-14-2110 PMID: 25593302
33. Oguma T et al (2009) Influence of black vinegar on itraconazole absorption Kansenshōgaku Zasshi 83(4) pp 369–74
34. Briasoulis E et al (2013) Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study BMC Cancer 13 pp 263 DOI: 10.1186/1471-2407-13-263 PMID: 23718900 PMCID: 3674943
35. Bhatt RS et al (2010) A phase 2 pilot trial of low-dose, continuous infusion, or “metronomic” paclitaxel and oral celecoxib in patients with metastatic melanoma Cancer 116(7) pp 1751–6 DOI: 10.1002/cncr.24902 PMID: 20120033 PMCID: 2847062
36. Doudican NA et al (2013) XIAP downregulation accompanies mebendazole growth inhibition in melanoma xenografts Anticancer Drugs 24(2) pp 181–8 DOI: 10.1097/CAD.0b013e32835a43f1
37. Nygren P and Larsson R (2013) Drug repositioning from bench to bedside: Tumour remission by the antihelmintic drug mebendazole in refractory metastatic colon cancer Acta Oncol 53(3) 427–8 DOI: 10.3109/0284186X.2013.844359
38. Bastiaannet E et al (2012) Use of Aspirin postdiagnosis improves survival for colon cancer patients Br J Cancer 106(9) 1532–1827 pp 1564–70
39. Barber EL et al (2013) The combination of intravenous bevacizumab and metronomic oral cyclophosphamide is an effective regimen for platinum-resistant recurrent ovarian cancer J Gynecol Oncol 24(3) pp 258–64 DOI: 10.3802/jgo.2013.24.3.258 PMID: 23875076 PMCID: 3714464
40. Niwa K et al (2008) Prognostic implications of cimetidine on advanced serous ovarian carcinoma related to cyclooxygenase-2 expression Molecular Med Rep 1(1) pp 119–22






