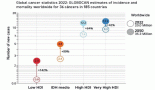
For oncology, one of the biggest effects of the reforms to the National Health Service (NHS) in England has been the designation of systemic anticancer treatments (other than hormonal agents) as a specialised service. This means that all decisions regarding the commissioning of chemotherapy are made at the national level via NHS England (NHSE), under expert guidance from the Chemotherapy Clinical Reference Group (CRG). Commissioning decisions will be based on several factors, not only clinical efficacy and cost/affordability, but also the ‘added value’ that a new treatment may offer in terms of patient outcomes, resource utilisation, and/or wider benefits to society. Oncology health-care professionals (HCPs) are in a position to affect cancer commissioning decisions in the reformed NHS, not only the small number who are members of the Chemotherapy CRG, but also as advisors to the Chemotherapy CRG via disease-specific consensus work, and through participation in the collection and reporting of real-life data on patient outcomes. With the emerging emphasis on both consensus work and outcomes data, a step change can also be expected in the relationship between HCPs and the pharmaceutical industry, including a strengthened role for non-promotional education, support for forums and consensus groups, and facilitation of the development, collection, and dissemination of findings from real-life practice. In addition, there will be an onus on the pharmaceutical industry to provide information on the implications that new products may have for service delivery and capacity and for meeting patients’ and society’s expectations. This information will need to be developed and delivered in a timely way, well in advance of the launch of a new product.